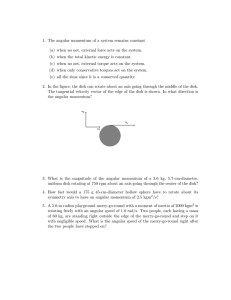
Problem set 13
... L (α is half the opening angle of the cone swept out by Ω). Express α in terms of θ , the principal moments of inertia and the magnitude of angular momentum L. How does α depend on time and L? (b) h3i Suppose I1 → I3 so that the symmetric top becomes a spherical top. Based on our study of the spheri ...
... L (α is half the opening angle of the cone swept out by Ω). Express α in terms of θ , the principal moments of inertia and the magnitude of angular momentum L. How does α depend on time and L? (b) h3i Suppose I1 → I3 so that the symmetric top becomes a spherical top. Based on our study of the spheri ...
Problem Set 7
... (b) From this result and the fact that the atomic mass of gold is 197u, where u is the atomic mass unit, compute a lower limit on the mass density of nuclear material. ...
... (b) From this result and the fact that the atomic mass of gold is 197u, where u is the atomic mass unit, compute a lower limit on the mass density of nuclear material. ...
7. DOMAIN OF VALIDITY OF CLASSICAL THEORY I1x I1px h. (7.1
... The right-hand side of this inequality is just the de Broglie wavelength of the reduced mass particle (except for an extra factor of 1j21t), and in wave terms, this is just the condition that diffraction effects be small. This inequality is generally satisfied in collisions involving heavy particles ...
... The right-hand side of this inequality is just the de Broglie wavelength of the reduced mass particle (except for an extra factor of 1j21t), and in wave terms, this is just the condition that diffraction effects be small. This inequality is generally satisfied in collisions involving heavy particles ...
WHAT IS INSIDE AN ATOM? - Florida State University
... Schrödinger equation: (Erwin Schrödinger, 1925) is a differential equation for matter waves; basically a formulation of energy conservation. its solution called “wave function”, usually denoted by ; |(x)|2 gives the probability of finding the particle at x; applied to the hydrogen atom, th ...
... Schrödinger equation: (Erwin Schrödinger, 1925) is a differential equation for matter waves; basically a formulation of energy conservation. its solution called “wave function”, usually denoted by ; |(x)|2 gives the probability of finding the particle at x; applied to the hydrogen atom, th ...
Assignment 1
... Show that (0, 0) is a center if and only if a + d = 0, ad − bc > 0. Show that the phase trajectories are then given by cx2 + 2dxy − by 2 = constant. 4. The relativistic linear harmonic oscillator is given by the equation of motion d m0 c2 (p ) + kx = 0, (k > 0, v = ẋ) dt 1 − v 2 /c2 ...
... Show that (0, 0) is a center if and only if a + d = 0, ad − bc > 0. Show that the phase trajectories are then given by cx2 + 2dxy − by 2 = constant. 4. The relativistic linear harmonic oscillator is given by the equation of motion d m0 c2 (p ) + kx = 0, (k > 0, v = ẋ) dt 1 − v 2 /c2 ...
Room: PHYS 238 Time: 9:00 10:15 Monday and Wednesday
... has developed in a natural progression since the turn of the last century These first two lectures attempt to provide the historical context and examples of the experiments that shape our current understanding of the most fundamental principles of nature. (based on a seminar given to the Purdue REU ...
... has developed in a natural progression since the turn of the last century These first two lectures attempt to provide the historical context and examples of the experiments that shape our current understanding of the most fundamental principles of nature. (based on a seminar given to the Purdue REU ...
QuestionSheet
... where v is the magnitude of the electron velocity and theta is the angle between its direction and the cobalt-60 spin. Deduce the value of the coefficient alpha by considering events in which the electron is emitted in the direction of the decaying nuclei. The spins of the cobalt and Nickel nuclei a ...
... where v is the magnitude of the electron velocity and theta is the angle between its direction and the cobalt-60 spin. Deduce the value of the coefficient alpha by considering events in which the electron is emitted in the direction of the decaying nuclei. The spins of the cobalt and Nickel nuclei a ...
Does the Third Law of Thermodynamics Hold
... Boltzmann entropy vanishes when the bath temperature is zero,” leading them to note “—the violation of the third law reported here for nonweak coupling.” On the contrary, we argue that all such calculations should lead to the same result so we are motivated to examine the question of what went wrong ...
... Boltzmann entropy vanishes when the bath temperature is zero,” leading them to note “—the violation of the third law reported here for nonweak coupling.” On the contrary, we argue that all such calculations should lead to the same result so we are motivated to examine the question of what went wrong ...
CHAPTER 3: The Experimental Basis of Quantum
... It approaches the data at longer wavelengths, but it deviates badly at short wavelengths. This problem for small wavelengths became known as the ultraviolet catastrophe and was one of the outstanding exceptions that classical physics could not explain. ...
... It approaches the data at longer wavelengths, but it deviates badly at short wavelengths. This problem for small wavelengths became known as the ultraviolet catastrophe and was one of the outstanding exceptions that classical physics could not explain. ...
The Quantum-Mechanical Model of the Atom
... • Note that width at halfheight of a spectrum line is written as δν. ...
... • Note that width at halfheight of a spectrum line is written as δν. ...
powerpoint
... Superposition creates regions of constructive and destructive diffraction according to the relative incidence of the waves. The light intensity is distributed by the square of the wave envelope: ...
... Superposition creates regions of constructive and destructive diffraction according to the relative incidence of the waves. The light intensity is distributed by the square of the wave envelope: ...
My Century of Physics
... At Caltech I gave the first postwar course in field theory based on what I had learned from the written presentations of Pauli and Wentzel. There I met and began to work with Mal Ruderman, and he became my first doctoral student. That year UCLA offered positions to Gamow and myself. Gamow was happy ...
... At Caltech I gave the first postwar course in field theory based on what I had learned from the written presentations of Pauli and Wentzel. There I met and began to work with Mal Ruderman, and he became my first doctoral student. That year UCLA offered positions to Gamow and myself. Gamow was happy ...
Physics Notes for Class 12 Chapter 12 Atoms
... (i) About the Stability of Atom According to Maxwell’s electromagnetic wave theory electron should emit energy in the form of electromagnetic wave during its orbital motion. Therefore. radius of orbit of electron will decrease gradually and ultimately it will fall in the nucleus. (ii) About the Line ...
... (i) About the Stability of Atom According to Maxwell’s electromagnetic wave theory electron should emit energy in the form of electromagnetic wave during its orbital motion. Therefore. radius of orbit of electron will decrease gradually and ultimately it will fall in the nucleus. (ii) About the Line ...