Grossmont College Chemistry 120 Laboratory Manual 6th Edition
... 5-mm cross rulings. Always have the data entry portion prepared in advance, and record data directly in your final report as you obtain it. (Data entered on scraps of paper will be confiscated.) Where calculations of data are involved, show an orderly calculation for the first set of data, but do no ...
... 5-mm cross rulings. Always have the data entry portion prepared in advance, and record data directly in your final report as you obtain it. (Data entered on scraps of paper will be confiscated.) Where calculations of data are involved, show an orderly calculation for the first set of data, but do no ...
Determination of Hydrogen Bond Structure in Water versus Aprotic
... that hydrogen bonds adopt shorter equilibrium distances in aprotic environments than in water and that such shortening deepens the potential energy well of the hydrogen-bonded groups and results in a much more favorable formation free energy (Figure 1C, additional discussion in Text S1).8,12,15−23 T ...
... that hydrogen bonds adopt shorter equilibrium distances in aprotic environments than in water and that such shortening deepens the potential energy well of the hydrogen-bonded groups and results in a much more favorable formation free energy (Figure 1C, additional discussion in Text S1).8,12,15−23 T ...
5 Energetics - Pearson Schools and FE Colleges
... dependent on the transfer of energy that occurs when fuels burn. As we explore the source of these energy changes, we will deepen our understanding of why bonds are broken and formed during a chemical reaction, and why electron transfer can lead to the formation of stable ionic compounds. The questi ...
... dependent on the transfer of energy that occurs when fuels burn. As we explore the source of these energy changes, we will deepen our understanding of why bonds are broken and formed during a chemical reaction, and why electron transfer can lead to the formation of stable ionic compounds. The questi ...
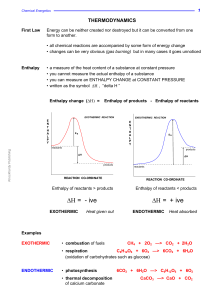
Η - Knockhardy
... the individual atoms join up again but in the form of products. The overall energy change will depend on the difference between the energy required to break the bonds and that released as bonds are made. energy released making bonds > energy used to break bonds ... EXOTHERMIC energy used to break bo ...
... the individual atoms join up again but in the form of products. The overall energy change will depend on the difference between the energy required to break the bonds and that released as bonds are made. energy released making bonds > energy used to break bonds ... EXOTHERMIC energy used to break bo ...
Low-Energy (20 eV) and High-Energy (1000 eV) Electron
... of several prebiotic species, is relatively abundant. The interactions of high-energy radiation, such as cosmic rays (Emax ~1020 eV), with matter produce large numbers of low-energy secondary electrons, which are known to initiate radiolysis reactions in the condensed phase. Using temperature progra ...
... of several prebiotic species, is relatively abundant. The interactions of high-energy radiation, such as cosmic rays (Emax ~1020 eV), with matter produce large numbers of low-energy secondary electrons, which are known to initiate radiolysis reactions in the condensed phase. Using temperature progra ...
2 CHEMICAL ARITHMATICS W MODULE - 1
... (please refer to lesson-1). Now, in order to study chemical compounds and reactions in the laboratory, it is necessary to have adequate knowledge of the quantitative relationship among the amounts of the reacting substances that take part and products formed in the chemical reaction. This relationsh ...
... (please refer to lesson-1). Now, in order to study chemical compounds and reactions in the laboratory, it is necessary to have adequate knowledge of the quantitative relationship among the amounts of the reacting substances that take part and products formed in the chemical reaction. This relationsh ...
Document
... pizzas, we burn a pizza, drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum nu ...
... pizzas, we burn a pizza, drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum nu ...
C - Wits Structural Chemistry
... molecule, there are only the three "horizontal" C2 axes and the planes bisect the angle between them. In the Newman projection the reason for calling these planes "dihedral" is clear. Finally we consider the complex in figure 1.15c where there are no "horizontal" C2 axes but there are two sets of pl ...
... molecule, there are only the three "horizontal" C2 axes and the planes bisect the angle between them. In the Newman projection the reason for calling these planes "dihedral" is clear. Finally we consider the complex in figure 1.15c where there are no "horizontal" C2 axes but there are two sets of pl ...
chapter - Max-Planck-Institut für Astronomie
... are injected into the gas-phase, where they are observed via their rotational lines. Complex organic molecules, precursors of prebiotic species, are also detected at this stage. ...
... are injected into the gas-phase, where they are observed via their rotational lines. Complex organic molecules, precursors of prebiotic species, are also detected at this stage. ...
Department of Chemistry
... Chemistry is the scientific study of the composition and properties of matter and the investigation of the laws that govern them. Classically, chemistry is divided into several subdisciplines. Organic chemistry deals primarily with carbon compounds; inorganic chemistry, with compounds of the other e ...
... Chemistry is the scientific study of the composition and properties of matter and the investigation of the laws that govern them. Classically, chemistry is divided into several subdisciplines. Organic chemistry deals primarily with carbon compounds; inorganic chemistry, with compounds of the other e ...
sample problem - KFUPM Resources
... More practical aspect of Gibb’s free energy • The standard free energy (ΔGrxn) of a system is the change in free energy when reactants in their standard states are converted to products in their standard states. • Standard states are: ...
... More practical aspect of Gibb’s free energy • The standard free energy (ΔGrxn) of a system is the change in free energy when reactants in their standard states are converted to products in their standard states. • Standard states are: ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... it also determines the amounts of the other ingredients we will use! ...
... it also determines the amounts of the other ingredients we will use! ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... pizzas, we burn a pizza, drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum nu ...
... pizzas, we burn a pizza, drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum nu ...
State the main methods used to prepare polymers?
... Vulcanization or vulcanisation refers to a specific curing process of rubber involving high heat and the addition of sulfur or other equivalent curatives. It is a chemical process in which polymer molecules are linked to other polymer molecules by atomic bridges composed of sulfur atoms or carbon to ...
... Vulcanization or vulcanisation refers to a specific curing process of rubber involving high heat and the addition of sulfur or other equivalent curatives. It is a chemical process in which polymer molecules are linked to other polymer molecules by atomic bridges composed of sulfur atoms or carbon to ...
OC 583- ISOTOPE BIGEOCHEMISTRY
... 2. How do they measure the absolute ratio of the standards? -Gravimetrically, but good only to about ±0.2‰ e.g., Baertschi, in Earth Planet. Sci. Lett. 31: 341-344. -However, the absolute isotope ratio of the standard isn’t that important because in most situations we are concerned with knowing the ...
... 2. How do they measure the absolute ratio of the standards? -Gravimetrically, but good only to about ±0.2‰ e.g., Baertschi, in Earth Planet. Sci. Lett. 31: 341-344. -However, the absolute isotope ratio of the standard isn’t that important because in most situations we are concerned with knowing the ...