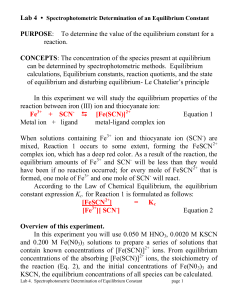
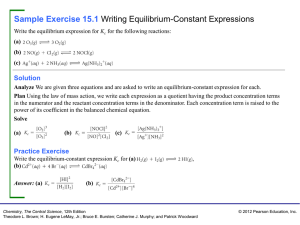
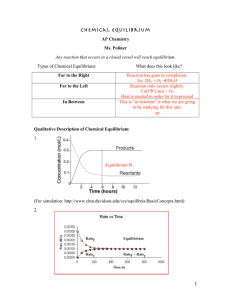
PURPOSE: To determine the value of the equilibrium constant for a
... Since the Fe3+ concentration is great excess in part A, this error would have no impact on the outcome. The SCN- concentration determines the concentration of FeSCN2+. 8. An error was made in preparing the KSCN solution in Part A. Its concentration was 0.003 molar but was labeled as 0.002 molar. How ...
... Since the Fe3+ concentration is great excess in part A, this error would have no impact on the outcome. The SCN- concentration determines the concentration of FeSCN2+. 8. An error was made in preparing the KSCN solution in Part A. Its concentration was 0.003 molar but was labeled as 0.002 molar. How ...
Organic Chemistry Notes
... Alkane compounds with halogen substituents (F, Cl, Br or I) Alkyl Halides Naming: .same manner as alkyl groups .attached F, Cl Br or I atoms called: fluoro, chloro, bromo and iodo groups .use number to indicate attachment position on hydrocarbon chain .if more than one of same kind of halogen is pre ...
... Alkane compounds with halogen substituents (F, Cl, Br or I) Alkyl Halides Naming: .same manner as alkyl groups .attached F, Cl Br or I atoms called: fluoro, chloro, bromo and iodo groups .use number to indicate attachment position on hydrocarbon chain .if more than one of same kind of halogen is pre ...
TDB-5: Standards and conventions for TDB publications
... the ligands. For example, (UO2 )2 CO3 (OH)− 3 is standard, (UO2 )2 (OH)3 CO3 is non-standard and should not be used. The treatment of organic ligands poses certain problems due to the complicated composition of most of them. A notation in conformity with the structural features of the molecule may t ...
... the ligands. For example, (UO2 )2 CO3 (OH)− 3 is standard, (UO2 )2 (OH)3 CO3 is non-standard and should not be used. The treatment of organic ligands poses certain problems due to the complicated composition of most of them. A notation in conformity with the structural features of the molecule may t ...
Chapter 5 ppt
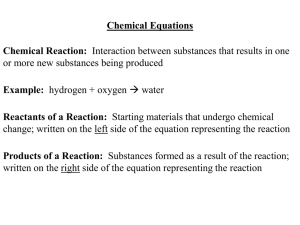
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen water ...
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen water ...
PDF File
... which the 2′-OH is replaced by -NH+3 (Table 1). The –NH+3 group has no lone pair electrons and therefore cannot interact with a metal ion. The rSNH+3 reaction would therefore be expected to be severely compromised if the 2′-OH coordinates a metal ion important for catalysis (e.g. [10]). To determine ...
... which the 2′-OH is replaced by -NH+3 (Table 1). The –NH+3 group has no lone pair electrons and therefore cannot interact with a metal ion. The rSNH+3 reaction would therefore be expected to be severely compromised if the 2′-OH coordinates a metal ion important for catalysis (e.g. [10]). To determine ...
Chemical Equations Chemical Reaction: Interaction between
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen Æ water Reactants of a Reaction: Starting materials that undergo chemical change; written on the left side of the equation representing the reaction Products of a Re ...
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen Æ water Reactants of a Reaction: Starting materials that undergo chemical change; written on the left side of the equation representing the reaction Products of a Re ...
chapter 16
... 1. Look for very small K values (where K < 10-5) , "x" may be negligible. You must check validity by plugging "x" over original concentration. It must be less than 5% of the original concentration to be valid. 2. If "x" is necessary, then see if the problem may be a perfect square and thus, ease the ...
... 1. Look for very small K values (where K < 10-5) , "x" may be negligible. You must check validity by plugging "x" over original concentration. It must be less than 5% of the original concentration to be valid. 2. If "x" is necessary, then see if the problem may be a perfect square and thus, ease the ...
Combustion and thermal degradation of polymers
... Photodegradation or light-induced polymer degradation, or, concerns the physical and chemical changes caused by irradiation of polymers with ultraviolet or visible light. In order to be effective, light must be absorbed by the substrate. Thus, the existence of chromophoric (light absorbing) groups ...
... Photodegradation or light-induced polymer degradation, or, concerns the physical and chemical changes caused by irradiation of polymers with ultraviolet or visible light. In order to be effective, light must be absorbed by the substrate. Thus, the existence of chromophoric (light absorbing) groups ...
THESE DOCTORAT DE L`UNIVERSITE DE TOULOUSE ET
... A puzzling difference in relative reactivity of the Mo and W systems (W >> Mo when using H2O2, W << Mo when using TBHP), however, remains without a satisfactory rationalization. A detailed investigations of the speciation behavior of the tungsten compound, [Cp*2W2O5], through combined 1H NMR and el ...
... A puzzling difference in relative reactivity of the Mo and W systems (W >> Mo when using H2O2, W << Mo when using TBHP), however, remains without a satisfactory rationalization. A detailed investigations of the speciation behavior of the tungsten compound, [Cp*2W2O5], through combined 1H NMR and el ...
The Mole
... Don't multiply the molar mass of a substance by the coefficient in the problem BEFORE using it in one of the steps above. For example, if the formula says 2 H2O, DON'T use 36.0 g/mol, use 18.0 g/mol. Don't round off until the very last answer. In other words, don't clear your calculator after step t ...
... Don't multiply the molar mass of a substance by the coefficient in the problem BEFORE using it in one of the steps above. For example, if the formula says 2 H2O, DON'T use 36.0 g/mol, use 18.0 g/mol. Don't round off until the very last answer. In other words, don't clear your calculator after step t ...
Chemistry 0310 - Organic Chemistry 1 Chapter 12. Reactions of
... - Syn-Hydroxylations of alkenes are most conveniently performed with catalytic OsO4 and NMO (N-methylmorpholine N-oxide) as co-oxidant. Attack occurs from the less-hindered face of the alkene, and a vicinal syn-diol is isolated after reductive workup. - Oxidative cleavage of 1,2-disubstituted alken ...
... - Syn-Hydroxylations of alkenes are most conveniently performed with catalytic OsO4 and NMO (N-methylmorpholine N-oxide) as co-oxidant. Attack occurs from the less-hindered face of the alkene, and a vicinal syn-diol is isolated after reductive workup. - Oxidative cleavage of 1,2-disubstituted alken ...
Rates of Reaction
... – If doubling the concentration had a quadrupling effect on the rate, one would deduce it was a second-order dependence. – A doubling of concentration that results in an eight-fold increase in the rate would be a thirdorder dependence. Copyright © Houghton Mifflin Company.All rights reserved. ...
... – If doubling the concentration had a quadrupling effect on the rate, one would deduce it was a second-order dependence. – A doubling of concentration that results in an eight-fold increase in the rate would be a thirdorder dependence. Copyright © Houghton Mifflin Company.All rights reserved. ...
Improved Synthesis, Separation, Transition Metal Coordination and
... P{1H} spectra of meso-Ni2Cl4(et,ph-P4) with 1-hexene in acetone-d6/D2O recorded at 15°C (light blue), 10°C (dark blue), 25°C (black), 50°C (orange), 80°C (purple), and 100°C (red). For higher temperatures the NMR tube was tube pressurized to 90 psi with O2 ......................................... ...
... P{1H} spectra of meso-Ni2Cl4(et,ph-P4) with 1-hexene in acetone-d6/D2O recorded at 15°C (light blue), 10°C (dark blue), 25°C (black), 50°C (orange), 80°C (purple), and 100°C (red). For higher temperatures the NMR tube was tube pressurized to 90 psi with O2 ......................................... ...
RSE on PVC «South Kazakhstan state pharmaceutical academy
... |ammonia |chloride ion |reducing materials ~ Impurity of nitrates and nitrites in purified water determined by reaction with …. |solution of diphenylamine in the environment of concentrated sulfuric acid |concentrated sulfuric acid |solution of potassium permanganate |solution of diphenylamine |solu ...
... |ammonia |chloride ion |reducing materials ~ Impurity of nitrates and nitrites in purified water determined by reaction with …. |solution of diphenylamine in the environment of concentrated sulfuric acid |concentrated sulfuric acid |solution of potassium permanganate |solution of diphenylamine |solu ...
Chemical Equilibrium - Chemistry with Mrs. Caruso Let the Bonding
... 2. At a particular temperature, K= 2.0 x 10-6 mol/L for the reaction 2CO2 (g) ⇌ 2CO (g) + O2 (g) If 2.0 mol of CO2 is initially placed into a 5.0-L vessel, calculate the equilibrium concentrations of all species. CO .0086M; O2.0043M; CO2 .39M 3. At 25°C, K= .090 for the reaction H2O (g) + Cl2O ( ...
... 2. At a particular temperature, K= 2.0 x 10-6 mol/L for the reaction 2CO2 (g) ⇌ 2CO (g) + O2 (g) If 2.0 mol of CO2 is initially placed into a 5.0-L vessel, calculate the equilibrium concentrations of all species. CO .0086M; O2.0043M; CO2 .39M 3. At 25°C, K= .090 for the reaction H2O (g) + Cl2O ( ...
Grossmont College Chemistry 120 Laboratory Manual 6th Edition
... 5-mm cross rulings. Always have the data entry portion prepared in advance, and record data directly in your final report as you obtain it. (Data entered on scraps of paper will be confiscated.) Where calculations of data are involved, show an orderly calculation for the first set of data, but do no ...
... 5-mm cross rulings. Always have the data entry portion prepared in advance, and record data directly in your final report as you obtain it. (Data entered on scraps of paper will be confiscated.) Where calculations of data are involved, show an orderly calculation for the first set of data, but do no ...