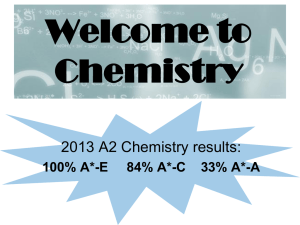
alcohols - GC12chem
... Secondary alcohol: the C atom with the OH group attached is attached to two other C atoms CH3CH2CHOHCH3 Tertiary alcohol: the C atom with the OH group attached is attached to three other C atoms CH3CH2C(CH3)OHCH3 12 Chemistry 2.5 organic chemistry CR 07 ...
... Secondary alcohol: the C atom with the OH group attached is attached to two other C atoms CH3CH2CHOHCH3 Tertiary alcohol: the C atom with the OH group attached is attached to three other C atoms CH3CH2C(CH3)OHCH3 12 Chemistry 2.5 organic chemistry CR 07 ...
Course No - Chemistry
... n-butanol , acetone and water (4:5:1); Spray reagent – aniline hydrogen phthalate. Qualitative analysis: Identification of single organic compound through functional group analysis and determination of melting point. C. Physical Chemistry : Transition Temperature: 1. Determination of the transition ...
... n-butanol , acetone and water (4:5:1); Spray reagent – aniline hydrogen phthalate. Qualitative analysis: Identification of single organic compound through functional group analysis and determination of melting point. C. Physical Chemistry : Transition Temperature: 1. Determination of the transition ...
C - b. finkel
... Isomers : compounds with identical molecular composition but their structures are arranged differently. Isomerism : another reason why there are so many organic compounds. ...
... Isomers : compounds with identical molecular composition but their structures are arranged differently. Isomerism : another reason why there are so many organic compounds. ...
Document
... complete outer shell consists of 8 valence electrons (except H and He which have 2) Destruction of a bond corresponds to a release of energy. Generally double or triple bond energies are higher than for single bonds. Ionic bonds: electron attraction between positive and negative ions e- transfer ...
... complete outer shell consists of 8 valence electrons (except H and He which have 2) Destruction of a bond corresponds to a release of energy. Generally double or triple bond energies are higher than for single bonds. Ionic bonds: electron attraction between positive and negative ions e- transfer ...
Full answers
... = (6.626 × 10–34 J s) × (2.998 × 108 m s-1) / (590 × 10-9 m) = 3.4 × 10–19 J The energy of 1 mol is therefore: E = (3.4 × 10–19 J) × (6.022 × 1023 mol–1) = 200 kJ mol–1 ...
... = (6.626 × 10–34 J s) × (2.998 × 108 m s-1) / (590 × 10-9 m) = 3.4 × 10–19 J The energy of 1 mol is therefore: E = (3.4 × 10–19 J) × (6.022 × 1023 mol–1) = 200 kJ mol–1 ...
- Fairview High School
... Key point: Rotation is impossible around a double bond. (Would require breaking pi bond) ...
... Key point: Rotation is impossible around a double bond. (Would require breaking pi bond) ...
COVENANT UNIVERSITY College of Science and Technology
... A selection of experiments designed to provide illustrations of the important parts of the lectures in CHM226 Course. The experiments will afford the students the opportunity to develop their quantitative and analytical skills. Topics include chemical equilibria, kinetics of iodination of acetone, d ...
... A selection of experiments designed to provide illustrations of the important parts of the lectures in CHM226 Course. The experiments will afford the students the opportunity to develop their quantitative and analytical skills. Topics include chemical equilibria, kinetics of iodination of acetone, d ...
level three chemistry: organics
... I can show that I understand the significance of the structure of each functional group by explaining the relative solubility of each functional group in terms of polarity and hydrogen bonding. I can show that I understand the significance of the structure of each functional group by explaining the ...
... I can show that I understand the significance of the structure of each functional group by explaining the relative solubility of each functional group in terms of polarity and hydrogen bonding. I can show that I understand the significance of the structure of each functional group by explaining the ...
School of Biomedical Biomolecular and Chemical Sciences
... Copying of this material by students, except for fair dealing purposes under the Copyright Act, is prohibited. For the purposes of this fair dealing exception, students should be aware that the rule allowing copying, for fair dealing purposes, of 10% of the work, or one chapter/article, applies to t ...
... Copying of this material by students, except for fair dealing purposes under the Copyright Act, is prohibited. For the purposes of this fair dealing exception, students should be aware that the rule allowing copying, for fair dealing purposes, of 10% of the work, or one chapter/article, applies to t ...
Organic Chemistry Practice Test
... A. Cellulose and Starch B. Polyethylene and Nylon C. Protein and Starch D. Protein and Nylon ...
... A. Cellulose and Starch B. Polyethylene and Nylon C. Protein and Starch D. Protein and Nylon ...
Document
... The energy released by forming products is GREATER than the energy required to break the reactant’s bonds It’s surroundings would feel hot, because heat (energy) is being released. ...
... The energy released by forming products is GREATER than the energy required to break the reactant’s bonds It’s surroundings would feel hot, because heat (energy) is being released. ...
Course File - Chemical Engineering and Applied Chemistry
... Describe the concepts which deal with aromaticity, aromatic compounds and their nomenclature. Explain the aldehydes and ketones, nucleophilic additions to the carbonyl group. Explain the general facts of the aldol reactions which are addition of enolate ions to aldehydes and ketones. Describ ...
... Describe the concepts which deal with aromaticity, aromatic compounds and their nomenclature. Explain the aldehydes and ketones, nucleophilic additions to the carbonyl group. Explain the general facts of the aldol reactions which are addition of enolate ions to aldehydes and ketones. Describ ...
Biology 2B-1 - secondary
... – Organic compounds are based on carbon and its unusual bonding characteristics, They all contain carbon, most organic compounds under natural circumstances are the product of biosynthesis – Inorganic substances are derived from nonliving material and lack carbon ...
... – Organic compounds are based on carbon and its unusual bonding characteristics, They all contain carbon, most organic compounds under natural circumstances are the product of biosynthesis – Inorganic substances are derived from nonliving material and lack carbon ...
The Chemistry of Biology Student carbon compounds
... 9. What three structural groups shown do all amino acids have in common? 10. There are 20 naturally-occurring amino acids, and each one only varies in the structure of the R side chain. Two amino acids are shown in Model 1. What are the R side chains in each? ...
... 9. What three structural groups shown do all amino acids have in common? 10. There are 20 naturally-occurring amino acids, and each one only varies in the structure of the R side chain. Two amino acids are shown in Model 1. What are the R side chains in each? ...
CYL110
... the very small intermolecular attractions of this atom. • Tc of the noble gas elements increases with atomic number. • Hydrogen gas cannot be liquified above 33 K; this poses a major difficulty in the use of hydrogen as an automotive fuel; storage as a high-pressure gas requires heavy steel containe ...
... the very small intermolecular attractions of this atom. • Tc of the noble gas elements increases with atomic number. • Hydrogen gas cannot be liquified above 33 K; this poses a major difficulty in the use of hydrogen as an automotive fuel; storage as a high-pressure gas requires heavy steel containe ...
Organic Chemistry Questions
... (a) What is the molecular formula for the compounds? (b) Draw the structural formulas for the four possible noncyclic isomers with this molecular formula. (c) In the presence of an appropriate catalyst, both gases add hydrogen. The hydrogenated products are identical, their molecular weight is 58. W ...
... (a) What is the molecular formula for the compounds? (b) Draw the structural formulas for the four possible noncyclic isomers with this molecular formula. (c) In the presence of an appropriate catalyst, both gases add hydrogen. The hydrogenated products are identical, their molecular weight is 58. W ...
5. Functional Groups
... are a characteristic feature of organic molecules that behave in a predictable way are composed of an atom or group of atoms are groups that replace a H in the corresponding alkane provide a way to classify families of organic compounds ...
... are a characteristic feature of organic molecules that behave in a predictable way are composed of an atom or group of atoms are groups that replace a H in the corresponding alkane provide a way to classify families of organic compounds ...