CHEM 203 Material
... Principle: determining whether the bonded state of a given atom resembles more closely the preceding or the successive noble gas allows one to make important predictions about chemical reactivity: Example: the C atom in CH4 has formally acquired 4 electrons, thereby assuming the oxidation state of – ...
... Principle: determining whether the bonded state of a given atom resembles more closely the preceding or the successive noble gas allows one to make important predictions about chemical reactivity: Example: the C atom in CH4 has formally acquired 4 electrons, thereby assuming the oxidation state of – ...
Multiwalled Boron Nitride Nanotubes: Growth, Properties, and
... highlighted in Sect. 3. Section 4 describes potential applications of BNNTs for polymer composites, electronic devices, and molecular biological and chemical applications. Readers are encouraged to read more about the experimental aspects of single-wall BNNTs in Chap. 3 and the related theory in Cha ...
... highlighted in Sect. 3. Section 4 describes potential applications of BNNTs for polymer composites, electronic devices, and molecular biological and chemical applications. Readers are encouraged to read more about the experimental aspects of single-wall BNNTs in Chap. 3 and the related theory in Cha ...
Chapter 25 Alt Notes 0910
... Because the -OH group is quite polar, the properties of alcohols depend upon the number of -OH groups per molecule and the size of the organic group. The boiling points of monohydric alcohols increase with increasing molecular weight. The solubility of monohydric alcohols in water decrease wit ...
... Because the -OH group is quite polar, the properties of alcohols depend upon the number of -OH groups per molecule and the size of the organic group. The boiling points of monohydric alcohols increase with increasing molecular weight. The solubility of monohydric alcohols in water decrease wit ...
Chapter 14: Alcohols, Phenols, and Ethers This chapter is the first of
... Ethers are Flammable, e.g., Diethyl ether has boiling point of 35oC and therefore a flash-fire hazard. Ethers react slowly with oxygen from the air to form unstable hydroperoxides and peroxides. Unreactive towards acids, bases and oxidizing agents (useful for organic reactions) Like alkanes, ethers ...
... Ethers are Flammable, e.g., Diethyl ether has boiling point of 35oC and therefore a flash-fire hazard. Ethers react slowly with oxygen from the air to form unstable hydroperoxides and peroxides. Unreactive towards acids, bases and oxidizing agents (useful for organic reactions) Like alkanes, ethers ...
Sample pages 6 PDF
... aldehydes, electronic effects can be very important, and oxidations yielding benzoic acids possessing electron donating groups can be much slower. • Somehow unexpectedly, the reaction speed decreases by increasing the temperature, a fact due to the decomposition of oxoammonium salts, which are very ...
... aldehydes, electronic effects can be very important, and oxidations yielding benzoic acids possessing electron donating groups can be much slower. • Somehow unexpectedly, the reaction speed decreases by increasing the temperature, a fact due to the decomposition of oxoammonium salts, which are very ...
Antonio Rizzo
... special nonlinear mixed electric and magnetic frequency dependent responses, conveniently computed nowadays employing modern analytic response theory tools. We will present, on the other hand, also other nonlinear chiroptical spectroscopic properties, all proven to be amenable to ab initio simulatio ...
... special nonlinear mixed electric and magnetic frequency dependent responses, conveniently computed nowadays employing modern analytic response theory tools. We will present, on the other hand, also other nonlinear chiroptical spectroscopic properties, all proven to be amenable to ab initio simulatio ...
applied sciences Chiral β-Amino Alcohols as Ligands for the N
... bonded to an activating group (aryl, alkoxy, amino, sulfinyl, sulfonyl or phosphinyl) are generally used in reduction methodologies leading to chiral amines. The phosphinyl group is very attractive since its removal from the reduction products can be conveniently achieved under mild acidic condition ...
... bonded to an activating group (aryl, alkoxy, amino, sulfinyl, sulfonyl or phosphinyl) are generally used in reduction methodologies leading to chiral amines. The phosphinyl group is very attractive since its removal from the reduction products can be conveniently achieved under mild acidic condition ...
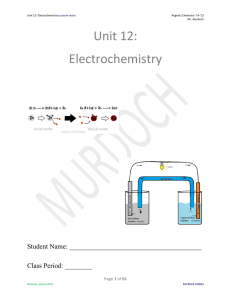
Unit 12: Electrochemistry
... 4. Converter: A device that takes AC commercial current and converts it to DC current at the step-down voltage required by the device. 5. Direct Current (DC): The current produced by generators and batteries, where electricity flows only from anode to cathode. DC current is used in battery-powered e ...
... 4. Converter: A device that takes AC commercial current and converts it to DC current at the step-down voltage required by the device. 5. Direct Current (DC): The current produced by generators and batteries, where electricity flows only from anode to cathode. DC current is used in battery-powered e ...
ch04 - alkanes
... Immiscible with water and other highly polar solvents Solvent for non -polar organic compounds t The eclipsed conformation has all C-H bonds on adjacent ...
... Immiscible with water and other highly polar solvents Solvent for non -polar organic compounds t The eclipsed conformation has all C-H bonds on adjacent ...
McMurray-Fay Chapter 22 Presentation Slides
... 2 Carbon Chain 3 Carbon Chain 4 Carbon Chain 5 Carbon Chain 6 Carbon Chain 7 Carbon Chain 8 Carbon Chain 9 Carbon Chain 10 Carbon Chain ...
... 2 Carbon Chain 3 Carbon Chain 4 Carbon Chain 5 Carbon Chain 6 Carbon Chain 7 Carbon Chain 8 Carbon Chain 9 Carbon Chain 10 Carbon Chain ...
Module 2
... The chemistry laboratory is not a dangerous place to work as long as all necessary precautions are taken seriously. In the following paragraphs, those important precautions are described. Everyone who works and performs experiments in a laboratory must follow these safety rules at all times. Student ...
... The chemistry laboratory is not a dangerous place to work as long as all necessary precautions are taken seriously. In the following paragraphs, those important precautions are described. Everyone who works and performs experiments in a laboratory must follow these safety rules at all times. Student ...
Chapter 8
... ◦ IUPAC nomenclature retains the common name aniline for C6H5NH2, the simplest aromatic amine. ◦ Name simple derivatives of aniline by using numbers to locate substituents or, alternatively, use the prefixes ortho (o), meta (m), and para (p). ◦ Several derivatives of aniline have common names that a ...
... ◦ IUPAC nomenclature retains the common name aniline for C6H5NH2, the simplest aromatic amine. ◦ Name simple derivatives of aniline by using numbers to locate substituents or, alternatively, use the prefixes ortho (o), meta (m), and para (p). ◦ Several derivatives of aniline have common names that a ...
Electrophilic Additions: Alkenes Addition of Hydrogen Halides
... the hydrogen adds to the carbon that has the greater number of hydrogen substituents, substituents, and the halogen adds to the carbon that has the fewer hydrogen substituents. substituents. ...
... the hydrogen adds to the carbon that has the greater number of hydrogen substituents, substituents, and the halogen adds to the carbon that has the fewer hydrogen substituents. substituents. ...
ppt
... 24.5: Substituent Effects on the Acidity of Phenols. Electron-donating substituents make a phenol less acidic by destabilizing the phenoxide ion (resonance effect) X ...
... 24.5: Substituent Effects on the Acidity of Phenols. Electron-donating substituents make a phenol less acidic by destabilizing the phenoxide ion (resonance effect) X ...
Chemistry: Percent Yield
... 17: 3.4e Equal volumes of gases at the same temperature and pressure contain an equal number of particles. 33: 3.2b Types of chemical reactions include synthesis, decomposition, single replacement, and double replacement 36: M1.1C – Use algebraic and geometric representations to describe and compare ...
... 17: 3.4e Equal volumes of gases at the same temperature and pressure contain an equal number of particles. 33: 3.2b Types of chemical reactions include synthesis, decomposition, single replacement, and double replacement 36: M1.1C – Use algebraic and geometric representations to describe and compare ...
OCR Gateway Science
... When molten lead iodide is electrolysed using a current of 12 A for 50 minutes, approximately 39 g of lead is deposited. (a) What mass of lead is produced using a current of 12 A for 25 minutes? (b) What mass of lead is produced using a current of 3 A for 50 minutes? (c) What charge in coulombs is t ...
... When molten lead iodide is electrolysed using a current of 12 A for 50 minutes, approximately 39 g of lead is deposited. (a) What mass of lead is produced using a current of 12 A for 25 minutes? (b) What mass of lead is produced using a current of 3 A for 50 minutes? (c) What charge in coulombs is t ...
Practice Exam 4
... Please refer to the cover page for needed thermodynamic values and formulas. 001 10.0 points An isolated system allows for the flow of...? 1. none of these correct 2. sound waves 3. kinetic energy 4. matter 5. heat Explanation: In the natural sciences an isolated system is a physical system without ...
... Please refer to the cover page for needed thermodynamic values and formulas. 001 10.0 points An isolated system allows for the flow of...? 1. none of these correct 2. sound waves 3. kinetic energy 4. matter 5. heat Explanation: In the natural sciences an isolated system is a physical system without ...