makeup2
... likely to react chemically to form ions which have a charge of (A) +2e (B) +e (C) -e (D) -2e 14. The formula of the compound ammonium carbonate is (A) NH4CO3 (B) NH4CO4 (C) NH4HCO3 (D) (NH4)2CO3 15. Which statement regarding the properties of elements arranged in the Periodic Table is correct? (A) A ...
... likely to react chemically to form ions which have a charge of (A) +2e (B) +e (C) -e (D) -2e 14. The formula of the compound ammonium carbonate is (A) NH4CO3 (B) NH4CO4 (C) NH4HCO3 (D) (NH4)2CO3 15. Which statement regarding the properties of elements arranged in the Periodic Table is correct? (A) A ...
Macromolecules
... The glucose that is not used immediately is converted in the liver and muscles into glycogen for storage. Any glucose in excess of the needs for energy and storage as glycogen is converted to fat. ...
... The glucose that is not used immediately is converted in the liver and muscles into glycogen for storage. Any glucose in excess of the needs for energy and storage as glycogen is converted to fat. ...
Document
... 38. ___Most of the metals found above hydrogen in the activity series are found as elements in the ground. 39. __ Gold is a highly reactive metal. 40. ___ Barium hydroxide produced in a double displacement reaction will precipitate out. 41. ___ Hydrogen is in the activity series because it classifie ...
... 38. ___Most of the metals found above hydrogen in the activity series are found as elements in the ground. 39. __ Gold is a highly reactive metal. 40. ___ Barium hydroxide produced in a double displacement reaction will precipitate out. 41. ___ Hydrogen is in the activity series because it classifie ...
Bonding 1. Which one of the following is most likely to be an ionic
... 7. Water has such a high specific heat because a. it has such a low molecular weight. b. it is rather dense. c. the O-H single bond has a high bond energy. d. it has many relatively strong hydrogen bonds. e. it dissolves both ionic and covalent compounds. ...
... 7. Water has such a high specific heat because a. it has such a low molecular weight. b. it is rather dense. c. the O-H single bond has a high bond energy. d. it has many relatively strong hydrogen bonds. e. it dissolves both ionic and covalent compounds. ...
CHEM IB Lecture notes as of 8-29-06
... Tuesday August 29th, 2006 - Day 2: Questions of the day: What is Chemistry? What are two types of scientific observations and what are ways of representing them graphically? SWBAT: Discuss the importance of chemistry & the two basic types of observations by making such observations What is Chemistry ...
... Tuesday August 29th, 2006 - Day 2: Questions of the day: What is Chemistry? What are two types of scientific observations and what are ways of representing them graphically? SWBAT: Discuss the importance of chemistry & the two basic types of observations by making such observations What is Chemistry ...
Fundamentals of Theoretical Organic Chemistry Lecture 1
... Some of the mathematical models given in Figure1.1.2-8, together with other formulas not shown on figure, can be combined to compute internal potential energies. The equations may be combined in a number of possible ways. The combined equations, collectively referred to as “force - fields”, incorpor ...
... Some of the mathematical models given in Figure1.1.2-8, together with other formulas not shown on figure, can be combined to compute internal potential energies. The equations may be combined in a number of possible ways. The combined equations, collectively referred to as “force - fields”, incorpor ...
Chapter 1
... • One way to understand this: AOs and MOs are wave functions (QM says e have wave characteristics). --Two AOs can overlap constructively (wave reinforcement) to form a ______________ MO. --Or they can overlap destructively (wave cancellation) to form an _________________________ MO. ...
... • One way to understand this: AOs and MOs are wave functions (QM says e have wave characteristics). --Two AOs can overlap constructively (wave reinforcement) to form a ______________ MO. --Or they can overlap destructively (wave cancellation) to form an _________________________ MO. ...
Preview Sample 1
... D) are always some form of carbohydrate. E) are naturally similar to sugars. 102) Alaska Natives have a lower incidence of heart disease even though their diets are high in fat and cholesterol. This may be due to the large amount of ________ in their diets. A) steroids B) omega-3 fatty acids C) trig ...
... D) are always some form of carbohydrate. E) are naturally similar to sugars. 102) Alaska Natives have a lower incidence of heart disease even though their diets are high in fat and cholesterol. This may be due to the large amount of ________ in their diets. A) steroids B) omega-3 fatty acids C) trig ...
H 2 and H 2 + O 2 g H 2 O and H 2 O Hydrogen + Oxygen g Water
... New substances are formed by chemical reactions – when elements react together to form compounds their atoms join to other atoms via chemical bonds Chemical bonds involve electrons from the reacting atoms – bonds can form when: Electrons are transferred from one atom to another, so that one at ...
... New substances are formed by chemical reactions – when elements react together to form compounds their atoms join to other atoms via chemical bonds Chemical bonds involve electrons from the reacting atoms – bonds can form when: Electrons are transferred from one atom to another, so that one at ...
CHEMICAL REACTIONS OBJECTIVES 1. To study reactions
... When sodium bicarbonate, e.g. baking soda, is combined with an acid, a gas is produced HCO3- + H+ -----> CO2(g) + H2O(l) ...
... When sodium bicarbonate, e.g. baking soda, is combined with an acid, a gas is produced HCO3- + H+ -----> CO2(g) + H2O(l) ...
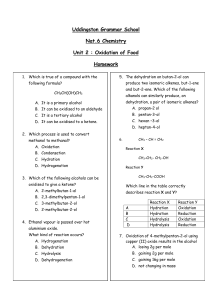
Unit 2 - Calderglen High School
... In the reaction, the carbon atom next to the carbonyl functional group of one molecule forms a bond with the carbonyl carbon atom of the second molecule. (a) Draw a structural formula for the product formed when propanone is used instead of ethanal in this type of reaction. (1) (b) Name an aldehyde ...
... In the reaction, the carbon atom next to the carbonyl functional group of one molecule forms a bond with the carbonyl carbon atom of the second molecule. (a) Draw a structural formula for the product formed when propanone is used instead of ethanal in this type of reaction. (1) (b) Name an aldehyde ...
Classifying Organic Molecules
... 12. Take your non-nitrogen pile and sort out those cards that have -OH attached to most carbons. Be aware that organic chemists use many shortcuts in drawing complex molecules. They often do not include the letter C for carbon in ring structures. How many cards did you find? 13. What are the two for ...
... 12. Take your non-nitrogen pile and sort out those cards that have -OH attached to most carbons. Be aware that organic chemists use many shortcuts in drawing complex molecules. They often do not include the letter C for carbon in ring structures. How many cards did you find? 13. What are the two for ...
Self Test Question - University of Illinois at Chicago
... e Amines (e) are the least acidic acids of the group drawn above: amide anions (R2N-) are the least stable of the conjugate bases drawn above since nitrogen, being less electronegative than oxygen, is less able to bear the negative charge. N-H bonds are also stronger than O-H bonds. a vs. c phenols ...
... e Amines (e) are the least acidic acids of the group drawn above: amide anions (R2N-) are the least stable of the conjugate bases drawn above since nitrogen, being less electronegative than oxygen, is less able to bear the negative charge. N-H bonds are also stronger than O-H bonds. a vs. c phenols ...
WEEK 7
... is that fats contain a hydrocarbon portion that often resembles long-chain alkanes or alkenes. Compounds classifies as aromatic contain a benzene ring or a system of these rings. These compounds, also known as ARENES, are called aromatic because the first ones discovered had pleasant odors. Examples ...
... is that fats contain a hydrocarbon portion that often resembles long-chain alkanes or alkenes. Compounds classifies as aromatic contain a benzene ring or a system of these rings. These compounds, also known as ARENES, are called aromatic because the first ones discovered had pleasant odors. Examples ...