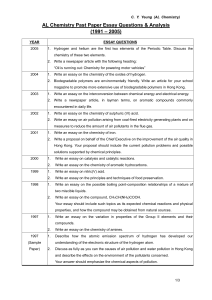
AL Chemistry Past paper essay questions
... Write an essay on amino acids, polypeptides and proteins. Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
... Write an essay on amino acids, polypeptides and proteins. Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
EVANS GROUP RESEARCH PROJECT DESCRIPTIONS
... synthesis of unusual hydrocarbon polymers. Since the lanthanide metals have not been heavily investigated in the past, they offer new options for polymer synthesis. Organolanthanide complexes have the added advantage that they can initiate polymerization without an aluminum co-catalyst such as MAO. ...
... synthesis of unusual hydrocarbon polymers. Since the lanthanide metals have not been heavily investigated in the past, they offer new options for polymer synthesis. Organolanthanide complexes have the added advantage that they can initiate polymerization without an aluminum co-catalyst such as MAO. ...
OME General Chemistry
... Hydrocarbons in Nature Natural gas is a mixture of low molecular weight hydrocarbons made up primarily of methane CH4, with lesser amounts of ethane C2H6, propane C3H8, and butane C4H10. Petroleum, or crude oil, is a mixture of alkanes and cycloalkanes with small amounts of aromatic hydrocarbons. F ...
... Hydrocarbons in Nature Natural gas is a mixture of low molecular weight hydrocarbons made up primarily of methane CH4, with lesser amounts of ethane C2H6, propane C3H8, and butane C4H10. Petroleum, or crude oil, is a mixture of alkanes and cycloalkanes with small amounts of aromatic hydrocarbons. F ...
Form A 1 Chem 130 Name______________________________
... 8. For the reaction, 2 NO(g) + Cl2(g) → 2 NOCl(g), Ho = -40.9 kJ. At what temperatures do you expect the reaction to be spontaneous: high, low, all, or none? Justify your answer. If we look at the reaction, there are three moles of gas on the reactant side and only two moles of gas on the product s ...
... 8. For the reaction, 2 NO(g) + Cl2(g) → 2 NOCl(g), Ho = -40.9 kJ. At what temperatures do you expect the reaction to be spontaneous: high, low, all, or none? Justify your answer. If we look at the reaction, there are three moles of gas on the reactant side and only two moles of gas on the product s ...
Lecture 15
... • Cells (plants and animals) rely on O2 for life processes – Water an electron acceptor in plants – Animal cells generate water from the reduction of O2 by H+ ...
... • Cells (plants and animals) rely on O2 for life processes – Water an electron acceptor in plants – Animal cells generate water from the reduction of O2 by H+ ...
AS Paper 1 Practice Paper 16 - A
... Explain why certain elements in the Periodic Table are classified as p-block elements. Illustrate your answer with an example of a p-block element and give its electronic configuration. ...
... Explain why certain elements in the Periodic Table are classified as p-block elements. Illustrate your answer with an example of a p-block element and give its electronic configuration. ...
CH100: Fundamentals for Chemistry
... The unit of Atomic Mass is the Dalton (formerly called the amu) 1 Dalton = one twelfth mass of one 12C atom = 1.661x10-27 kg Note: There 6 protons & 6 neutrons in a 12C atom but the mass of a 12C atom is actually less than the combined mass of all of the nucleons ...
... The unit of Atomic Mass is the Dalton (formerly called the amu) 1 Dalton = one twelfth mass of one 12C atom = 1.661x10-27 kg Note: There 6 protons & 6 neutrons in a 12C atom but the mass of a 12C atom is actually less than the combined mass of all of the nucleons ...
Honors Chemistry Semester 1 Exam Review
... State the Law of Conservation of Mass and explain its relationship to stoichiometry. ___________________________________________ ________________________________________________________________________________________________________________ __________________________________________________________ ...
... State the Law of Conservation of Mass and explain its relationship to stoichiometry. ___________________________________________ ________________________________________________________________________________________________________________ __________________________________________________________ ...
Methods S2.
... Fluorescence polarization binding assays (FP). All FP experiments were performed as described by Czarna et al. (1). Briefly, the fluorescence polarization experiments were read on an Ultra Evolution 384-well plate reader (Tecan) with the 485 nm excitation and 535 nm emission filters. The fluorescenc ...
... Fluorescence polarization binding assays (FP). All FP experiments were performed as described by Czarna et al. (1). Briefly, the fluorescence polarization experiments were read on an Ultra Evolution 384-well plate reader (Tecan) with the 485 nm excitation and 535 nm emission filters. The fluorescenc ...
Question Paper - Revision Science
... Complete the equation for the reaction between the hydrochloric acid in the toilet cleaner and the chloric(I) acid in the bleaching agent. Give a reason why this reaction is to be avoided in accordance with the instructions for the use of the toilet cleaner. ...
... Complete the equation for the reaction between the hydrochloric acid in the toilet cleaner and the chloric(I) acid in the bleaching agent. Give a reason why this reaction is to be avoided in accordance with the instructions for the use of the toilet cleaner. ...
Honors BIOLOGY - Mrs. Loyd`s Biology
... (CH2O)n where n = 3 → 8. A six-carbon monosaccharide would be C6H12O6. ...
... (CH2O)n where n = 3 → 8. A six-carbon monosaccharide would be C6H12O6. ...
Experiment 4 - Macalester College
... The three-dimensionality of a molecule is critically linked to its chemical and physical properties. For example, the bent geometry of water causes its polarity. The polarity of a molecule (or lack thereof) strongly influences its boiling point, freezing point, reactivity, and countless other proper ...
... The three-dimensionality of a molecule is critically linked to its chemical and physical properties. For example, the bent geometry of water causes its polarity. The polarity of a molecule (or lack thereof) strongly influences its boiling point, freezing point, reactivity, and countless other proper ...
Solute - St John Brebeuf
... compounds together when adding a solute to a solvent to make a solution…bonds need to break and new bonds need to form! So, we need to remember the intermolecular forces that hold molecules together…. ...
... compounds together when adding a solute to a solvent to make a solution…bonds need to break and new bonds need to form! So, we need to remember the intermolecular forces that hold molecules together…. ...
... considered the use of microwave (MW) irradiation as a source of energy. One-pot multicomponent coupling reactions (MCRs), where several organic moieties are coupled in one step, for carbon-carbon and carbon–heteroatom bond formation is an attractive synthetic strategy for the synthesis of small–mole ...
File
... • The covalent bond between two carbon atoms is strong so that the backbones are stable. In all of these compounds simple sub-units called monomers are linked together by condensation reactions. ...
... • The covalent bond between two carbon atoms is strong so that the backbones are stable. In all of these compounds simple sub-units called monomers are linked together by condensation reactions. ...
chemical reactions
... CHEMISTRY AND LIFE One unromantic but productive way of viewing life is to see it as a set of coordinated chemical reactions. ...
... CHEMISTRY AND LIFE One unromantic but productive way of viewing life is to see it as a set of coordinated chemical reactions. ...
Matter
... Each element has its own set of properties: how it looks, how hard it is, how well it conducts electricity and how it reacts with other elements. ...
... Each element has its own set of properties: how it looks, how hard it is, how well it conducts electricity and how it reacts with other elements. ...
chemistry
... The last page of the booklet is the answer sheet. Fold the last page along the perforations and, slowly and carefully, tear off the answer sheet. Then fill in the heading of your answer sheet. All of your answers are to be recorded on the separate answer sheet. For each question, decide which of the ...
... The last page of the booklet is the answer sheet. Fold the last page along the perforations and, slowly and carefully, tear off the answer sheet. Then fill in the heading of your answer sheet. All of your answers are to be recorded on the separate answer sheet. For each question, decide which of the ...