Organic Chemistry
... Carboxylic acids contain the carboxyl ( −COOH ) group. • A Carboxyl group is a carbonyl next to a hydroxyl group • All amino acids have a carboxylic acid used in peptide linking. • Have low pKa and deprotonate easily. ...
... Carboxylic acids contain the carboxyl ( −COOH ) group. • A Carboxyl group is a carbonyl next to a hydroxyl group • All amino acids have a carboxylic acid used in peptide linking. • Have low pKa and deprotonate easily. ...
Bimolecular reactions of the chromium
... The study of the gas-phase reactions of bare transition-metal cations with organic substrates during the last few years has given a wealth.of information about organometallic chemistry.’ A typical gas-phase reaction of a transition-metal ion with a hydrocarbon is the oxidative insertion into C-H and ...
... The study of the gas-phase reactions of bare transition-metal cations with organic substrates during the last few years has given a wealth.of information about organometallic chemistry.’ A typical gas-phase reaction of a transition-metal ion with a hydrocarbon is the oxidative insertion into C-H and ...
Organic and Biological Molecules
... Carbon readily forms long chains of bonds with itself. This property is called catenation, and is fairly unique. It results for several reasons: 1. Carbon can make up to 4 bonds. 2. The carbon-carbon bond is generally as strong as bonds between carbon and other ...
... Carbon readily forms long chains of bonds with itself. This property is called catenation, and is fairly unique. It results for several reasons: 1. Carbon can make up to 4 bonds. 2. The carbon-carbon bond is generally as strong as bonds between carbon and other ...
Developing Lewis structures 1) Add up all the valence electrons for
... for each negative charge; subtract one electron for each positive charge. 2) Connect the atoms using lines to represent bonding pairs of electrons. The central atom is usually that wich is least electronegative. (In most organic molecules, the carbons atoms make up the frame of the molecule.) 3) Any ...
... for each negative charge; subtract one electron for each positive charge. 2) Connect the atoms using lines to represent bonding pairs of electrons. The central atom is usually that wich is least electronegative. (In most organic molecules, the carbons atoms make up the frame of the molecule.) 3) Any ...
10. IJHAMS - ROLE OF CHEMISTRY IN EVERYDAY LIFE 1
... of water changes immediately, it surely means that the tea has been dyed with some water soluble colour. If iron filling is present in tea they can be removed by moving a magnet through the sample. Due to pollution, fluoride and aluminum are also sometimes present in tea. (13) Milk: Milk and milk pr ...
... of water changes immediately, it surely means that the tea has been dyed with some water soluble colour. If iron filling is present in tea they can be removed by moving a magnet through the sample. Due to pollution, fluoride and aluminum are also sometimes present in tea. (13) Milk: Milk and milk pr ...
chemistry
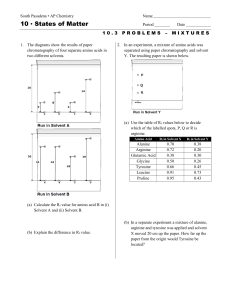
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Spring 2014
... (8 pts) If it takes 4.184 J of energy to raise the temperature of exactly one gram of water one degree Celcius, how many photons from this LED are needed to raise the temperature of 250 g of water (about one cup) one degree Celcius? ...
... (8 pts) If it takes 4.184 J of energy to raise the temperature of exactly one gram of water one degree Celcius, how many photons from this LED are needed to raise the temperature of 250 g of water (about one cup) one degree Celcius? ...
Part I Power generation in fuel cells
... problem is to use a large surface area for the electrode. Although in such cells the normal oxidizing fuel is oxygen, because of its easy availability, the fuel for the anode reaction may be any of a wide variety of materials. The hydrocarbons form a particularly important group of anode fuels. Hydr ...
... problem is to use a large surface area for the electrode. Although in such cells the normal oxidizing fuel is oxygen, because of its easy availability, the fuel for the anode reaction may be any of a wide variety of materials. The hydrocarbons form a particularly important group of anode fuels. Hydr ...
Chemical Reactions
... elements) combine and form a compound. (Sometimes these are called combination or addition reactions.) ...
... elements) combine and form a compound. (Sometimes these are called combination or addition reactions.) ...
Chemistry - Edexcel
... centre number and candidate number. all questions. t Answer the questions in the spaces provided t Answer – there may be more space than you need. Show all the steps in any calculations and state the units. t Some questions must be answered with a cross in a box t your mind about an answer, put a li ...
... centre number and candidate number. all questions. t Answer the questions in the spaces provided t Answer – there may be more space than you need. Show all the steps in any calculations and state the units. t Some questions must be answered with a cross in a box t your mind about an answer, put a li ...
Document
... the lifeless, primordial Earth. As shown in this recreation, Miller used electrical discharges (simulated lightning) to trigger reactions in a primitive “atmosphere” of H2O, H2, NH3 (ammonia), and CH4 (methane)—some of the gases released by volcanoes ...
... the lifeless, primordial Earth. As shown in this recreation, Miller used electrical discharges (simulated lightning) to trigger reactions in a primitive “atmosphere” of H2O, H2, NH3 (ammonia), and CH4 (methane)—some of the gases released by volcanoes ...
Chemistry
... Dr Vogel’s classic introduction to analytical methods has provided generations of chemists worldwide with a basis for teaching, learning and applying analytical chemistry. This 60th anniversary edition - the first for a decade - reflects major changes in the subject. Analysts need to understand the ...
... Dr Vogel’s classic introduction to analytical methods has provided generations of chemists worldwide with a basis for teaching, learning and applying analytical chemistry. This 60th anniversary edition - the first for a decade - reflects major changes in the subject. Analysts need to understand the ...
Chem C1403 Lecture 6. Lewis structures and the geometry of
... (1) Compute the number of valence electrons from the composition. Add one more electron for each negative charge in the composition. Subtract one electron for each positive charge in the composition. (2) All acceptable Lewis structures must have the correct composition of atoms and charge. (3) An at ...
... (1) Compute the number of valence electrons from the composition. Add one more electron for each negative charge in the composition. Subtract one electron for each positive charge in the composition. (2) All acceptable Lewis structures must have the correct composition of atoms and charge. (3) An at ...
AP Chemistry - cloudfront.net
... 9.29 Using the periodic table only arrange the members of each of the following sets in order of increasing bond strength. (a) Br-Br, Cl-Cl, I-I; (b) S-H, S-Br, S-Cl; (c) C== N, C-N, C==N. 9.41 Using EN values, indicate the polarity of the following bonds with polar arrows, and determine the more po ...
... 9.29 Using the periodic table only arrange the members of each of the following sets in order of increasing bond strength. (a) Br-Br, Cl-Cl, I-I; (b) S-H, S-Br, S-Cl; (c) C== N, C-N, C==N. 9.41 Using EN values, indicate the polarity of the following bonds with polar arrows, and determine the more po ...
Organic Chemistry
... that has all its carbons connected in a row. • Branched chain alkanes: An alkane that has a branching connection of carbons. • Isomers: Compounds with same molecular formula but different structures. ...
... that has all its carbons connected in a row. • Branched chain alkanes: An alkane that has a branching connection of carbons. • Isomers: Compounds with same molecular formula but different structures. ...
Lecture 6
... Important: the term “molecular geometry” refers to the positions of the atoms in space about the central atom. Any lone pairs that are on the central atom are not considered in describing the final geometry. Thus, the SAME electronic geometry (same steric number) may correspond to more than one mol ...
... Important: the term “molecular geometry” refers to the positions of the atoms in space about the central atom. Any lone pairs that are on the central atom are not considered in describing the final geometry. Thus, the SAME electronic geometry (same steric number) may correspond to more than one mol ...
Synthesis of Imidazolium Room-Temperature Ionic
... pure by 1H NMR. Several purification protocols can be utilized to remove colored impurities either at the stage of the bromide or later (10), if spectroscopic grade ionic liquids are desired. Carrying out the reaction in a solvent ensures that the resulting ionic liquid does not develop a brown colo ...
... pure by 1H NMR. Several purification protocols can be utilized to remove colored impurities either at the stage of the bromide or later (10), if spectroscopic grade ionic liquids are desired. Carrying out the reaction in a solvent ensures that the resulting ionic liquid does not develop a brown colo ...
Welcome to AP Chemistry! AP Chemistry is
... Know that elements with similar properties are placed into columns (called groups) in the periodic table. Define and distinguish between metals, nonmetals, and metalloids. Identify main-group and transition elements on the periodic table. Know the general properties of elements in some speci ...
... Know that elements with similar properties are placed into columns (called groups) in the periodic table. Define and distinguish between metals, nonmetals, and metalloids. Identify main-group and transition elements on the periodic table. Know the general properties of elements in some speci ...