The Chemical Basis of Life
... Isotopes are atoms of an element that all have the same number of protons, but have different numbers of neutrons. Radioisotopes – radioactive decay The time it takes for half of a radioactive substance to decay is called its half-life. ...
... Isotopes are atoms of an element that all have the same number of protons, but have different numbers of neutrons. Radioisotopes – radioactive decay The time it takes for half of a radioactive substance to decay is called its half-life. ...
Chapters 14
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
Molecular Biology I
... 3 Use knowledge of supporting basic coures to understand molecular biology 1 processes. 4 Understand and apply basic techniques in gene technology, molecular biology and 1 genetics. 5 Understand the importance of techniques and modelsystems in molecular biology. ...
... 3 Use knowledge of supporting basic coures to understand molecular biology 1 processes. 4 Understand and apply basic techniques in gene technology, molecular biology and 1 genetics. 5 Understand the importance of techniques and modelsystems in molecular biology. ...
Balancing Equations Notes
... Balancing Equations Chemical Equation: a way to represent chemical reactions on paper. Animation http://www.chemistry.ohio-state.edu/betha/nealChemBal/ ...
... Balancing Equations Chemical Equation: a way to represent chemical reactions on paper. Animation http://www.chemistry.ohio-state.edu/betha/nealChemBal/ ...
Introduction to Chemistry and Measurement
... close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun. ...
... close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun. ...
SC71 Chemistry
... dependent variables when appropriate. Select feasible equipment and materials for labs. Create data tables and graphs from data collected. Evaluating data collected in graphical form; use a graph of mass versus volume to find the density; use density to solve problems related to mass and volume. Det ...
... dependent variables when appropriate. Select feasible equipment and materials for labs. Create data tables and graphs from data collected. Evaluating data collected in graphical form; use a graph of mass versus volume to find the density; use density to solve problems related to mass and volume. Det ...
Program Review - Austin Community College
... support. Lab safety and ease of operation would be greatly improved with a larger technical staff. The Chemistry Department would like to have more technological support. We want more computers and room for the computers. In conjunction, we would like to have programs that simulate concepts taught i ...
... support. Lab safety and ease of operation would be greatly improved with a larger technical staff. The Chemistry Department would like to have more technological support. We want more computers and room for the computers. In conjunction, we would like to have programs that simulate concepts taught i ...
Chem 150 - Fall 2015 Exam I
... b. One ounce of ethanol weights 28.4 g. If 331 kcal of heat are released per mole of ethanol that undergoes combustion, how many kcal of heat are released from the burning of 2 ½ ounces of ...
... b. One ounce of ethanol weights 28.4 g. If 331 kcal of heat are released per mole of ethanol that undergoes combustion, how many kcal of heat are released from the burning of 2 ½ ounces of ...
NYS Regents Chemistry June 21, 2002
... 2: III. MOLE/STOICHIOMETRY\5. Math and Chemical Equations\D. Mole-Mole Problems\1. Mole - Mole Problems - (15, 37) 1: IV. CHEMICAL BONDING\2. Bond Types\C. Metallic Bonding / Properties\1. Metallic Bonding / Properties - (8) 1: IV. CHEMICAL BONDING\2. Bond Types\A. Ionic Bonding / Properties\1. Ioni ...
... 2: III. MOLE/STOICHIOMETRY\5. Math and Chemical Equations\D. Mole-Mole Problems\1. Mole - Mole Problems - (15, 37) 1: IV. CHEMICAL BONDING\2. Bond Types\C. Metallic Bonding / Properties\1. Metallic Bonding / Properties - (8) 1: IV. CHEMICAL BONDING\2. Bond Types\A. Ionic Bonding / Properties\1. Ioni ...
1st Olympiad of Metropolises Chemistry Theoretical Problems
... the Gibbs energy of photosynthesis is 480 kJ/mol of CO2; green plants absorb ~10% of the available solar energy; 25% of the absorbed energy is used for the photosynthesis process. ...
... the Gibbs energy of photosynthesis is 480 kJ/mol of CO2; green plants absorb ~10% of the available solar energy; 25% of the absorbed energy is used for the photosynthesis process. ...
FYBSc Revised Syllabus
... 2.5.2. Acetylation of amines with acetic anhydride and acetyl chloride, Action of nitrous acid on primary, secondary and tertiary amines, Methylation of primary, secondary and tertiary amines, yielding quaternary ammonium salts; Hoffmann elimination. Note: Each reaction should be studied with respec ...
... 2.5.2. Acetylation of amines with acetic anhydride and acetyl chloride, Action of nitrous acid on primary, secondary and tertiary amines, Methylation of primary, secondary and tertiary amines, yielding quaternary ammonium salts; Hoffmann elimination. Note: Each reaction should be studied with respec ...
General Chemistry I - University of Toledo
... sequence. The parallel lab courses are CHEM 1280 and CHEM 1290, which you may be taking with the lecture, but it is not required that you do so. This sequence is appropriate for students who are majoring in the natural sciences, science education, pharmacy, engineering and some allied health fields. ...
... sequence. The parallel lab courses are CHEM 1280 and CHEM 1290, which you may be taking with the lecture, but it is not required that you do so. This sequence is appropriate for students who are majoring in the natural sciences, science education, pharmacy, engineering and some allied health fields. ...
Chemistry Standard Course of Study -- Detailed - UNCG GK-12
... Explain how metallic bonding determines the characteristics of metals: high MP, high BP, high conductivity, malleability, ductility, and luster. Students should be able to: ...
... Explain how metallic bonding determines the characteristics of metals: high MP, high BP, high conductivity, malleability, ductility, and luster. Students should be able to: ...
Picobiology
... acid residues of proteins. This means that the structural basis for a specific reaction to occur is clarified. However, the resolution of 10 pm is not sufficient to unveil the black box. In order to unveil the black box, vibrational spectroscopic analysis is required as well. It provides us with str ...
... acid residues of proteins. This means that the structural basis for a specific reaction to occur is clarified. However, the resolution of 10 pm is not sufficient to unveil the black box. In order to unveil the black box, vibrational spectroscopic analysis is required as well. It provides us with str ...
Chapter 4 Notes
... • Energies of atoms are fixed and definite quantities • Energy transitions occur in jumps of discrete amounts of energy • Electrons only lose energy when they move to a lower energy state ...
... • Energies of atoms are fixed and definite quantities • Energy transitions occur in jumps of discrete amounts of energy • Electrons only lose energy when they move to a lower energy state ...
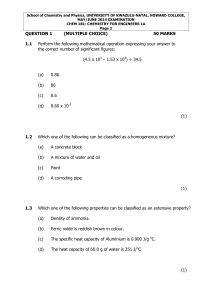
School of Chemistry
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...