Basic Laboratory Materials Science and Engineering Scanning Electron Microscopy
... intake of secondary electrons of low energy. Between collector and scintillator, high voltage of 10 kV is applied, accelerating the SE to come forcibly into contact with the scintillator. The scintillator consists either of a glass plate coated with luminescent powder (phosphor compound) or of a YAG ...
... intake of secondary electrons of low energy. Between collector and scintillator, high voltage of 10 kV is applied, accelerating the SE to come forcibly into contact with the scintillator. The scintillator consists either of a glass plate coated with luminescent powder (phosphor compound) or of a YAG ...
Honors Midterm Review – 2015-16
... _________ responsible for the uncertainty principle which states that it is impossible to know (with any great degree of certainty) both the location and velocity of an electron) _________ responsible for the planetary model of the atom, where electrons traveled in distinct paths around the nucleus ...
... _________ responsible for the uncertainty principle which states that it is impossible to know (with any great degree of certainty) both the location and velocity of an electron) _________ responsible for the planetary model of the atom, where electrons traveled in distinct paths around the nucleus ...
Sugárkémiai áttekintés Schiller Róbert
... This was written in 1907. Ramsay thought it was a question of total energy. Others compared water radiolysis with electrolysis ...
... This was written in 1907. Ramsay thought it was a question of total energy. Others compared water radiolysis with electrolysis ...
Answers for Review Questions Exam 3
... 10. Electrolysis is used as a source of elements from their ions. Ex. Na from Molten NaCl, Cl2 from a NaCl solution. 11. 0.1663 A current is needed. 12. First 2.47 Volts should be 2.47 Amperes. That gives 4.100g of Fe deposited. 13. Corrosion is the loss of metals to a solution of some form. The pro ...
... 10. Electrolysis is used as a source of elements from their ions. Ex. Na from Molten NaCl, Cl2 from a NaCl solution. 11. 0.1663 A current is needed. 12. First 2.47 Volts should be 2.47 Amperes. That gives 4.100g of Fe deposited. 13. Corrosion is the loss of metals to a solution of some form. The pro ...
doc
... photocell so that the coated black surface is facing the mercury lamp. Do not attach the cover yet. 4. Mount the iris diaphragm (b) on the optical bench at the marked position using an optical rider (H = 120 mm). 5. Mount the lens (c) at the marked position using an optical rider (H = 120 mm), revol ...
... photocell so that the coated black surface is facing the mercury lamp. Do not attach the cover yet. 4. Mount the iris diaphragm (b) on the optical bench at the marked position using an optical rider (H = 120 mm). 5. Mount the lens (c) at the marked position using an optical rider (H = 120 mm), revol ...
Midterm Review.ppt - Chemistry R: 4(AE)
... • Which halogens are gases at STP? 1. chlorine and fluorine 2. chlorine and bromine 3. iodine and fluorine ...
... • Which halogens are gases at STP? 1. chlorine and fluorine 2. chlorine and bromine 3. iodine and fluorine ...
Slide 1
... chemical properties of a sample of unknown gas and then investigated the gas. Which statement represents a conclusion rather than an ...
... chemical properties of a sample of unknown gas and then investigated the gas. Which statement represents a conclusion rather than an ...
Trends in the periodic table - Brigham Young University
... • Shielding effect of core electrons (S) • Nuclear effective charge, Zeff • Zeff = Z – S – What is Z? What is S? ...
... • Shielding effect of core electrons (S) • Nuclear effective charge, Zeff • Zeff = Z – S – What is Z? What is S? ...
Exam 3 Review - Iowa State University
... d. Atomic radius, K or Cs e. Atomic radius, Se or Br 12. List three properties that distinguish nonmetals from metals. 13. Which of the following are solids at room temperature, and which are gases? a. CO2 b. BaO c. CuO d. F2 e. NO 14. Which substances are ionic and which are covalent? a. Br2 b. KO2 ...
... d. Atomic radius, K or Cs e. Atomic radius, Se or Br 12. List three properties that distinguish nonmetals from metals. 13. Which of the following are solids at room temperature, and which are gases? a. CO2 b. BaO c. CuO d. F2 e. NO 14. Which substances are ionic and which are covalent? a. Br2 b. KO2 ...
Physics 201: Experiment #5 – Electron Diffraction
... characteristic of particles then, perhaps, particles should also exhibit wave properties. He went on to say that the wavelength of a particle is inversely proportional to its momentum. With accurate measurements of the mass and charge of the electron (as ...
... characteristic of particles then, perhaps, particles should also exhibit wave properties. He went on to say that the wavelength of a particle is inversely proportional to its momentum. With accurate measurements of the mass and charge of the electron (as ...
Review Sheet Filled Out
... List the number of facts you know about electrons. Electrons closest to the nucleus have the least amount of energy Electrons farthest away from the nucleus have the most energy – valence e Have a negative charge Have insignificant mass and volume Reside in the 99.996% of the atom outside t ...
... List the number of facts you know about electrons. Electrons closest to the nucleus have the least amount of energy Electrons farthest away from the nucleus have the most energy – valence e Have a negative charge Have insignificant mass and volume Reside in the 99.996% of the atom outside t ...
Glowing Tubes for Signs, Television Sets, and Computers
... a blue glow appears. The presence of krypton gives an intense white light. A television picture tube or computer monitor is also fundamentally a cathode ray tube. In this case the electrons are directed onto a screen containing chemical compounds that glow when struck by fast-moving electrons. The u ...
... a blue glow appears. The presence of krypton gives an intense white light. A television picture tube or computer monitor is also fundamentally a cathode ray tube. In this case the electrons are directed onto a screen containing chemical compounds that glow when struck by fast-moving electrons. The u ...
Chem 101A Exam 4 Concepts Chapter 7 – Modern Atomic Theory
... Chem 101A Exam 4 Concepts Chapter 7 – Modern Atomic Theory Use formulas that relate energy of photon, frequency, wavelength, speed of light, and the Rydberg Equation Notable scientists and their contributions: Rutherford, Bohr, Planc, de Broglie, Heisenberg, Schrödinger. The four Quantum ...
... Chem 101A Exam 4 Concepts Chapter 7 – Modern Atomic Theory Use formulas that relate energy of photon, frequency, wavelength, speed of light, and the Rydberg Equation Notable scientists and their contributions: Rutherford, Bohr, Planc, de Broglie, Heisenberg, Schrödinger. The four Quantum ...
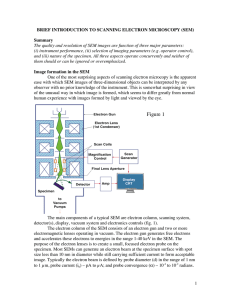
Introduction to Scanning Electron Microscopy (SEM)
... low aberrations, and the sample size is not limited by the lens gap. The aberrations of the final lens and consequently the resolution are controlled by a final lens aperture, which affects the beam convergence angle. The final lens aperture has three important effects on the final probe. First ther ...
... low aberrations, and the sample size is not limited by the lens gap. The aberrations of the final lens and consequently the resolution are controlled by a final lens aperture, which affects the beam convergence angle. The final lens aperture has three important effects on the final probe. First ther ...
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? ...
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? ...
Photoelectric effect
... the lamp passes through a grating monochromator, which can be adjusted to emit a beam of light at any wavelength from 350-700nm (and its higher energy harmonics - if you don’t know why, remind yourself how a diffraction grating works). The light then passes through a chopper that is connected to a ...
... the lamp passes through a grating monochromator, which can be adjusted to emit a beam of light at any wavelength from 350-700nm (and its higher energy harmonics - if you don’t know why, remind yourself how a diffraction grating works). The light then passes through a chopper that is connected to a ...
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 13. A sphere of gold has a radius of 1.5 inches. The volume of a sphere is 4.19r3. Given that the mass of this sphere is 4469 g, calculate the density of gold in g/cm3 to the correct number of significant figures. V = 4.19(1.5 in)3 =113.13 in3 x (2.54 cm/ in)3 = 231.73 cm3 ρ = 4469 g/ 231.73 cm3 = 1 ...
... 13. A sphere of gold has a radius of 1.5 inches. The volume of a sphere is 4.19r3. Given that the mass of this sphere is 4469 g, calculate the density of gold in g/cm3 to the correct number of significant figures. V = 4.19(1.5 in)3 =113.13 in3 x (2.54 cm/ in)3 = 231.73 cm3 ρ = 4469 g/ 231.73 cm3 = 1 ...
Gaseous detection device
The gaseous detection device-GDD is a method and apparatus for the detection of signals in the gaseous environment of an environmental scanning electron microscope (ESEM) and all scanned beam type of instruments that allow a minimum gas pressure for the detector to operate.