Unit 1, Lecture 1
... They are negatively charged. They have a spin (either up or down). The shapes of s and p orbitals s orbitals are spherically symmetric (“round”). p orbitals have two lobes with opposite sign along the axes. p orbitals are also triply degenerate. Atomic energy levels and electron configurations (firs ...
... They are negatively charged. They have a spin (either up or down). The shapes of s and p orbitals s orbitals are spherically symmetric (“round”). p orbitals have two lobes with opposite sign along the axes. p orbitals are also triply degenerate. Atomic energy levels and electron configurations (firs ...
Solid - burgess
... 1. different substances that are simply mixed together 2. can be separated by physical means (such as filtration, distillation, and chromatography) 3. Two types i. heterogeneous-does not have uniform composition; individual substances remain distinct. Examples are colloids and suspensions such as mu ...
... 1. different substances that are simply mixed together 2. can be separated by physical means (such as filtration, distillation, and chromatography) 3. Two types i. heterogeneous-does not have uniform composition; individual substances remain distinct. Examples are colloids and suspensions such as mu ...
Semester Exam Review Guide
... a. shows electrons in orbits similar to planets b. shows regions where electrons can be found c. shows probable areas where electrons are more likely to be located d. both b and c 21. The following model is a representation of the a. Electron cloud model b. Bohr’s model c. Democritus model d. John D ...
... a. shows electrons in orbits similar to planets b. shows regions where electrons can be found c. shows probable areas where electrons are more likely to be located d. both b and c 21. The following model is a representation of the a. Electron cloud model b. Bohr’s model c. Democritus model d. John D ...
april test
... Explain, in detail, whether you expect carbon tetrachloride (CCl4) to be polar or nonpolar (Hint. Your answer must include Lewis structures, molecular geometry and net dipole moment). ...
... Explain, in detail, whether you expect carbon tetrachloride (CCl4) to be polar or nonpolar (Hint. Your answer must include Lewis structures, molecular geometry and net dipole moment). ...
1 Light Microscopy
... where λ is the wavelength of light. From this it is clear that a good resolution (small δ) is connected with a high numerical aperture. The ability to distinguish two close points as distinct points is called Resolving power or Limit of resolution Limit of resolution of light microscope Lm = 0.16 / ...
... where λ is the wavelength of light. From this it is clear that a good resolution (small δ) is connected with a high numerical aperture. The ability to distinguish two close points as distinct points is called Resolving power or Limit of resolution Limit of resolution of light microscope Lm = 0.16 / ...
1. Which of the following statements best describes the
... Gas particles are packed closely together, but have some ability to move. ...
... Gas particles are packed closely together, but have some ability to move. ...
study guide first semester chemistry
... 8. Write a nuclear equation for the beta decay of 223Fr. ...
... 8. Write a nuclear equation for the beta decay of 223Fr. ...
Unit 9 – Behavior of Gases
... a. oxygen atom b. silicon atom 5. a. Calculate the wavelength, in meters, of green light that has a frequency of 5.0x1014 s-1? What is the energy of this light? (h = 6.626 x 10-34 Js) 6. What happens when an electron drops from a higher to a lower energy level? 7. Where do you find the following on ...
... a. oxygen atom b. silicon atom 5. a. Calculate the wavelength, in meters, of green light that has a frequency of 5.0x1014 s-1? What is the energy of this light? (h = 6.626 x 10-34 Js) 6. What happens when an electron drops from a higher to a lower energy level? 7. Where do you find the following on ...
P6.1.4.1 - LD Didactic
... The voltage of the capacitor can be influenced by induction effects. Move this part as little as possible during the experiment Dirt on the photocell can cause leakage currents between the anode and cathode which can effect the measurement of the limit voltage U0. Clean the photocell with alcohol. F ...
... The voltage of the capacitor can be influenced by induction effects. Move this part as little as possible during the experiment Dirt on the photocell can cause leakage currents between the anode and cathode which can effect the measurement of the limit voltage U0. Clean the photocell with alcohol. F ...
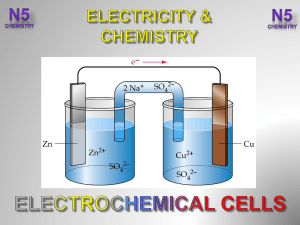
3.-Electrochemical-Cells-V2-
... The first small rechargeable battery produced for appliances, used a reaction between cadmium and a compound of nickel (nickel oxyhydroxide NiOOH) to produce a current. ...
... The first small rechargeable battery produced for appliances, used a reaction between cadmium and a compound of nickel (nickel oxyhydroxide NiOOH) to produce a current. ...
Principles of TEM image formation Principles of TEM image
... Phase-contrast microscope Wavefronts passing through the annulus illuminate the specimen and either pass through undeviated or are diffracted and retarded in phase by structures and phase gradients present in the specimen. Undeviated and diffracted light collected by the objective is segregated at ...
... Phase-contrast microscope Wavefronts passing through the annulus illuminate the specimen and either pass through undeviated or are diffracted and retarded in phase by structures and phase gradients present in the specimen. Undeviated and diffracted light collected by the objective is segregated at ...
stage iii – learning plan - Woodland Hills School District
... describe the wave characteristics of light. recognize and describe the key phenomena leading to the particle description of light use the mathematical relationships between the speed, frequency, wavelength, and energy of light. describe the key concepts of the Bohr Model and its explanation of t ...
... describe the wave characteristics of light. recognize and describe the key phenomena leading to the particle description of light use the mathematical relationships between the speed, frequency, wavelength, and energy of light. describe the key concepts of the Bohr Model and its explanation of t ...
CHE 1401 - Fall 2013 - Chapter 7 Homework 7 (Chapter 7: Periodic
... A) alkali metals have lower densities B) alkali metals have greater electron affinities C) alkali metals have lower ionization energies D) alkali metals have lower melting points E) alkali metals are not more reactive than alkaline earth metals ...
... A) alkali metals have lower densities B) alkali metals have greater electron affinities C) alkali metals have lower ionization energies D) alkali metals have lower melting points E) alkali metals are not more reactive than alkaline earth metals ...
The Periodic Table - Mrs Molchany`s Webpage
... across the period. More protons are added going across the period. The protons have a stronger pull on the electrons. The strong attractive forces between the protons and the outermost (valence) electrons shrinks the orbitals and makes the atoms smaller. Generally speaking, effective nuclear charge ...
... across the period. More protons are added going across the period. The protons have a stronger pull on the electrons. The strong attractive forces between the protons and the outermost (valence) electrons shrinks the orbitals and makes the atoms smaller. Generally speaking, effective nuclear charge ...
dyanmics and radiation of anelectrons driven by relativistically
... The radiation emitted by the electron is a set of extremely short pulses with attosecond durations, that is, pulses several orders of magnitude shorter than the driving laser pulse optical cycle. In the relativistic case, the compression is explained by the fact that the electron moves along the str ...
... The radiation emitted by the electron is a set of extremely short pulses with attosecond durations, that is, pulses several orders of magnitude shorter than the driving laser pulse optical cycle. In the relativistic case, the compression is explained by the fact that the electron moves along the str ...
Gaseous detection device
The gaseous detection device-GDD is a method and apparatus for the detection of signals in the gaseous environment of an environmental scanning electron microscope (ESEM) and all scanned beam type of instruments that allow a minimum gas pressure for the detector to operate.