AP Chem Stoichiometry Notes Table of Contents Atomic Masses
... o Often chemicals are mixed in exact quantities, so all of the reactants “run out” at the same time Stoichiometric quantities Haber Process Ammonia a very important starting material for the production of fertilizer. N2(g) + 3H2(g) 2NH3(g) o The hydrogen is made from the reaction of methane ...
... o Often chemicals are mixed in exact quantities, so all of the reactants “run out” at the same time Stoichiometric quantities Haber Process Ammonia a very important starting material for the production of fertilizer. N2(g) + 3H2(g) 2NH3(g) o The hydrogen is made from the reaction of methane ...
1 - JustAnswer
... 39. Solve using multiplication principle. Don't forget to perform a check. - 1/2x = - 7/8 (Simplify your answer. Type an integer or a fraction.) x = 7/4 40. Graph the equation and identify the y-intercept. y + x = - 2. (Type an ordered pair.) ...
... 39. Solve using multiplication principle. Don't forget to perform a check. - 1/2x = - 7/8 (Simplify your answer. Type an integer or a fraction.) x = 7/4 40. Graph the equation and identify the y-intercept. y + x = - 2. (Type an ordered pair.) ...
Equations of Perpendicular Lines
... line y = −3x + 4. Step 1 Find the slope of the perpendicular line. The graph of the given equation has a slope of −3. Because the slopes of perpendicular lines are negative reciprocals, the slope of the perpendicular line passing through (−3, 8) is —13. Step 2 Use the slope m = —13 and the slope-int ...
... line y = −3x + 4. Step 1 Find the slope of the perpendicular line. The graph of the given equation has a slope of −3. Because the slopes of perpendicular lines are negative reciprocals, the slope of the perpendicular line passing through (−3, 8) is —13. Step 2 Use the slope m = —13 and the slope-int ...
Category 3 - Denton ISD
... • Example: Mr. Jones charges $25 for an estimate plus $18 per hour to repair dents in car doors. The total charge for a repair is $151. How many hours did Mr. Jones work on the repair? 151 = 18h + 25 ...
... • Example: Mr. Jones charges $25 for an estimate plus $18 per hour to repair dents in car doors. The total charge for a repair is $151. How many hours did Mr. Jones work on the repair? 151 = 18h + 25 ...
Write this into your supplemental packet opposite page
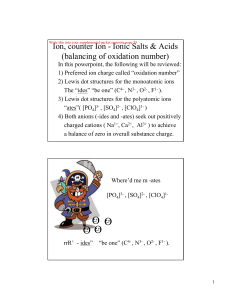
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
Lesson 6 - Perfect Squares and Factoring
... that it takes about 1.48 seconds for the ball to hit the ground. Example 5 Use the Square Root Property to Solve Equations Solve each equation. Check your solutions. a. (x – 2) 2 = ...
... that it takes about 1.48 seconds for the ball to hit the ground. Example 5 Use the Square Root Property to Solve Equations Solve each equation. Check your solutions. a. (x – 2) 2 = ...
Algebra Review
... form (y = mx + b) 2. Graph each equation on the same graph 3. Find where the lines intersect. This is your solution. a. If the lines are parallel “no solution” b. If the lines are the same “infinitely many solutions”. ...
... form (y = mx + b) 2. Graph each equation on the same graph 3. Find where the lines intersect. This is your solution. a. If the lines are parallel “no solution” b. If the lines are the same “infinitely many solutions”. ...
3.1 Atomic Mass - Pace University Webspace
... • A chemical reaction is a process in which a substance is changed into one or more new substances. • Chemical reactions are represented in a chemical equation to show what happens during such a reaction. • In a chemical equation, the substances that are the starting materials are called the reactan ...
... • A chemical reaction is a process in which a substance is changed into one or more new substances. • Chemical reactions are represented in a chemical equation to show what happens during such a reaction. • In a chemical equation, the substances that are the starting materials are called the reactan ...
Part II - American Chemical Society
... a score on Part II. Testing materials, scratch paper, and the “Blue Book” should be made available to the student only during the examination period. All testing materials including scratch paper should be turned in and kept secure until April 23, 2008, after which tests can be returned to students ...
... a score on Part II. Testing materials, scratch paper, and the “Blue Book” should be made available to the student only during the examination period. All testing materials including scratch paper should be turned in and kept secure until April 23, 2008, after which tests can be returned to students ...
Stoichiometry Worksheet #4
... 1. Silver sulfide (Ag2S) is the common tarnish on silver objects. What weight of silver sulfide can be made from 1.23 g of hydrogen sulfide (H2S) obtained from a rotten egg? The reaction of formation of silver sulfide is given below: Ag(s) + H2S(g) + O2(g) Ag2S(s) + H2O(l) (Equation must first be b ...
... 1. Silver sulfide (Ag2S) is the common tarnish on silver objects. What weight of silver sulfide can be made from 1.23 g of hydrogen sulfide (H2S) obtained from a rotten egg? The reaction of formation of silver sulfide is given below: Ag(s) + H2S(g) + O2(g) Ag2S(s) + H2O(l) (Equation must first be b ...
Document
... electron from the outer level. The nonmetal (chlorine) needs to gain one more to fill its outer level, and will accept the one electron that sodium is going to lose. ...
... electron from the outer level. The nonmetal (chlorine) needs to gain one more to fill its outer level, and will accept the one electron that sodium is going to lose. ...