Review Package
... 5. Ionic Compounds (Textbook p. 139-146; 148-149) Terminology (ion, cation, anion, ionic charge/combining capacity, valence electron, stable octet, polyatomic ion, binary compound, ternary compound, ionic bond) Draw Bohr-Rutherford diagrams/Lewis Dot structures to show the formation of ionic com ...
... 5. Ionic Compounds (Textbook p. 139-146; 148-149) Terminology (ion, cation, anion, ionic charge/combining capacity, valence electron, stable octet, polyatomic ion, binary compound, ternary compound, ionic bond) Draw Bohr-Rutherford diagrams/Lewis Dot structures to show the formation of ionic com ...
9 Electricity Notes
... • Open Circuit: a circuit in which there is a break in the wire so that current cannot flow,; a switch turned to the "off" position is one way to cause the break in the wire • Closed Circuit: a circuit in which the switch is turned to the "on" position, causing there to be no breaks anywhere in the ...
... • Open Circuit: a circuit in which there is a break in the wire so that current cannot flow,; a switch turned to the "off" position is one way to cause the break in the wire • Closed Circuit: a circuit in which the switch is turned to the "on" position, causing there to be no breaks anywhere in the ...
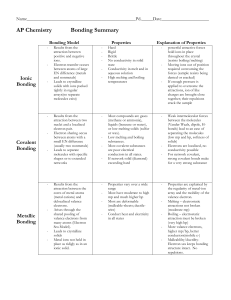
Types of Bonding Summary
... delocalized valence electrons. Arises through the shared pooling of valence electrons from many atoms (Electron Sea Model). Leads to crystalline solids Metal ions not held in place as ridigly as in an ionic solid. ...
... delocalized valence electrons. Arises through the shared pooling of valence electrons from many atoms (Electron Sea Model). Leads to crystalline solids Metal ions not held in place as ridigly as in an ionic solid. ...
Types of Chemical Reactions
... There are many types of chemical reactions. Five of the most common are: synthesis: two or more reactants combine to form a single product. A+BC decomposition: one reactant disintegrates (decomposes) to form two or more products: AB+C single replacement (sometimes called single displacement): atom ...
... There are many types of chemical reactions. Five of the most common are: synthesis: two or more reactants combine to form a single product. A+BC decomposition: one reactant disintegrates (decomposes) to form two or more products: AB+C single replacement (sometimes called single displacement): atom ...
Review for SNC 2P Chemistry Unit(SPRING 2014)
... bubble and a gas is released into the room. The following results are obtained… mass of reactants - ...
... bubble and a gas is released into the room. The following results are obtained… mass of reactants - ...
Physical Chemistry of Colloids and Surfaces – Final Exam Review 4-30-02
... ε ε ζ (Smoluchowski limit, high κR) u= r 0 µ Physically speaking, the Smoluchowski limit assumes the particles are forces indirectly by the electric field. The field pulls EDL ions, which drag solvent along with them. The resulting shear stress is transferred to the particle. ...
... ε ε ζ (Smoluchowski limit, high κR) u= r 0 µ Physically speaking, the Smoluchowski limit assumes the particles are forces indirectly by the electric field. The field pulls EDL ions, which drag solvent along with them. The resulting shear stress is transferred to the particle. ...
Electric Circuits
... current represents how many electrons pass a certain point in a certain amount of time. ...
... current represents how many electrons pass a certain point in a certain amount of time. ...
Introduction to Chemical Reactions and Equations Study Guide
... Compound Formulas Review 1. How do you know if a compound is ionic or covalent? Ionic – bond between a metal and nonmetal. Covalent – bond between a nonmetal and a nonmetal. ...
... Compound Formulas Review 1. How do you know if a compound is ionic or covalent? Ionic – bond between a metal and nonmetal. Covalent – bond between a nonmetal and a nonmetal. ...
Chemical Bonding Notes for 2016
... • Metals do not combine with metals. • They form alloys which is a solution of a metal in a metal. • Examples are steel, brass, bronze and pewter. ...
... • Metals do not combine with metals. • They form alloys which is a solution of a metal in a metal. • Examples are steel, brass, bronze and pewter. ...
Electric Potential
... It is the work needed to bring a unit positive charge from infinity to the given point. ...
... It is the work needed to bring a unit positive charge from infinity to the given point. ...
Physics with Mathematica Fall 2013 Exercise #4 17 Sep 2012
... Consider a straight line segment of uniformly distributed charge Q and length L, lying along the x-axis and centered on the origin. The line charge density is then simply λ = Q/L. Find the electrostatic potential along the z-axis, that is V (x) = V (0, 0, z). Express your result in the simplest form ...
... Consider a straight line segment of uniformly distributed charge Q and length L, lying along the x-axis and centered on the origin. The line charge density is then simply λ = Q/L. Find the electrostatic potential along the z-axis, that is V (x) = V (0, 0, z). Express your result in the simplest form ...
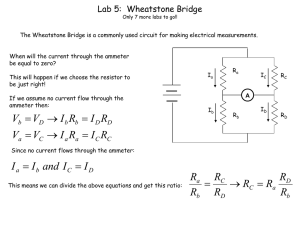
Powerpoint Slides
... The Wheatstone Bridge is a commonly used circuit for making electrical measurements. When will the current through the ammeter be equal to zero? This will happen if we choose the resistor to be just right! If we assume no current flow through the ammeter then: ...
... The Wheatstone Bridge is a commonly used circuit for making electrical measurements. When will the current through the ammeter be equal to zero? This will happen if we choose the resistor to be just right! If we assume no current flow through the ammeter then: ...
Chemical Nomenclature, Formulas, and Equations
... Strategy Our guide for writing formulas for ionic compounds is electrical neutrality; that is, the total charge on the cation(s) must be equal to the total charge on the anion(s). Because the charges on the Mg2+ and N3− ions are not equal, we know the formula cannot be MgN. Instead, we write the for ...
... Strategy Our guide for writing formulas for ionic compounds is electrical neutrality; that is, the total charge on the cation(s) must be equal to the total charge on the anion(s). Because the charges on the Mg2+ and N3− ions are not equal, we know the formula cannot be MgN. Instead, we write the for ...
ENGR 111 Teaching plan
... Voltage is the potential difference of charge at two points in an electrical field Voltage symbol V, unit Volts Voltage results in the flow of charge between two points ...
... Voltage is the potential difference of charge at two points in an electrical field Voltage symbol V, unit Volts Voltage results in the flow of charge between two points ...
U4D2 03.09 Notes
... • Voltage can come from a battery or a generator • We will look mostly at direct current (DC) circuits • Current flow will be in one direction • Voltage will be provided by a battery ...
... • Voltage can come from a battery or a generator • We will look mostly at direct current (DC) circuits • Current flow will be in one direction • Voltage will be provided by a battery ...
1) - Kurt Niedenzu
... 32) The increase in atomic radius of each successive element within a group is primarily due to an increase in the number of a) neutrons in the nucleus b) electrons in the outermost shell c) unpaired electrons d) occupied principal energy levels 33) Elements that have properties of both metals and n ...
... 32) The increase in atomic radius of each successive element within a group is primarily due to an increase in the number of a) neutrons in the nucleus b) electrons in the outermost shell c) unpaired electrons d) occupied principal energy levels 33) Elements that have properties of both metals and n ...
Physical Properties
... strong and thus require high energy to break. • Ion-ion attractions are stronger than the intermolecular forces: H-bonding, dipoledipole, and van der Waals’ forces. ...
... strong and thus require high energy to break. • Ion-ion attractions are stronger than the intermolecular forces: H-bonding, dipoledipole, and van der Waals’ forces. ...
lecture 5 revised
... Why use a spectroscopic technique for electrical characterisation? dc measurements give "total" or "composite" response: ...
... Why use a spectroscopic technique for electrical characterisation? dc measurements give "total" or "composite" response: ...
Test - Chemical Bonding- Practice Test
... ____ 29. the force of attraction between a positive and negative charge ____ 30. the element oxygen will gain two electrons to form a(n) ___________ ____ 31. the arrangement of electrons around the nucleus of an atom in its ground state ____ 32. atom or group of atoms having a positive charge ____ 3 ...
... ____ 29. the force of attraction between a positive and negative charge ____ 30. the element oxygen will gain two electrons to form a(n) ___________ ____ 31. the arrangement of electrons around the nucleus of an atom in its ground state ____ 32. atom or group of atoms having a positive charge ____ 3 ...
I. Why Atoms Combine - Manchester High School
... • most atoms form bonds in order to have 8 valence e• full outer energy level Ne • like the Noble Gases! Stability is the driving force behind bond ...
... • most atoms form bonds in order to have 8 valence e• full outer energy level Ne • like the Noble Gases! Stability is the driving force behind bond ...
Ohms Law - Powerpoint Presentation
... Electrical Potential is the energy stored ready to do work. It is measured in volts, is represented as V, and is determined by the source in a circuit. Electrical Flow is the flow of energy from a high potential point to a low potential point. This flow is called the current, is measured in ampere ...
... Electrical Potential is the energy stored ready to do work. It is measured in volts, is represented as V, and is determined by the source in a circuit. Electrical Flow is the flow of energy from a high potential point to a low potential point. This flow is called the current, is measured in ampere ...
Nanofluidic circuitry
Nanofluidic circuitry is a nanotechnology aiming for control of fluids in nanometer scale. Due to the effect of an electrical double layer within the fluid channel, the behavior of nanofluid is observed to be significantly different compared with its microfluidic counterparts. Its typical characteristic dimensions fall within the range of 1–100 nm. At least one dimension of the structure is in nanoscopic scale. Phenomena of fluids in nano-scale structure are discovered to be of different properties in electrochemistry and fluid dynamics.