Synthesis of Alum Lab
... 2Al(s) + 2K+(aq) + 2OH-(aq) + 6H2O(l) 2[Al(OH)4]-(aq) + 2K+(aq) + 3H2(g) The oxidation and reduction half reactions: ...
... 2Al(s) + 2K+(aq) + 2OH-(aq) + 6H2O(l) 2[Al(OH)4]-(aq) + 2K+(aq) + 3H2(g) The oxidation and reduction half reactions: ...
Title - Iowa State University
... b) K+, Cl- & O2c) K+ & ClO4- d) KClO4 9) Carbon disulfide (CS2), sugar (C6H12O6) and pure water are examples of a) strong electrolytes b) Weak electrolytes c) non-electrolytes d)base 10) Which one of these produces the largest number of particles per mole of dissolved solute in water? a) NaCl b) C2H ...
... b) K+, Cl- & O2c) K+ & ClO4- d) KClO4 9) Carbon disulfide (CS2), sugar (C6H12O6) and pure water are examples of a) strong electrolytes b) Weak electrolytes c) non-electrolytes d)base 10) Which one of these produces the largest number of particles per mole of dissolved solute in water? a) NaCl b) C2H ...
Physics - cloudfront.net
... • Remember the point charges we did the other day? • If two charges q1 and q2 are 1 meter apart, and have a charge of 1 Coulomb each, with what force will q1 push on q2? ...
... • Remember the point charges we did the other day? • If two charges q1 and q2 are 1 meter apart, and have a charge of 1 Coulomb each, with what force will q1 push on q2? ...
Electricity and Magnetism
... there will be a potential difference or voltage (V) created across the resistance. Ohm’s law gives a relationship between the voltage (V), current (I), and resistance (R) as follows: ...
... there will be a potential difference or voltage (V) created across the resistance. Ohm’s law gives a relationship between the voltage (V), current (I), and resistance (R) as follows: ...
3.0 Principles of Electrical Engineering.docx
... Alternating current (AC) is bi-directional, meaning that the flow of charge changes direction periodically5. As shown in Figure 2, the magnitude and direction of the current are not constant. From period t0 to t1 the current is positive and the flow in the circuit is clockwise. From period t1 to t2 ...
... Alternating current (AC) is bi-directional, meaning that the flow of charge changes direction periodically5. As shown in Figure 2, the magnitude and direction of the current are not constant. From period t0 to t1 the current is positive and the flow in the circuit is clockwise. From period t1 to t2 ...
Electrical Engineering
... and bends if current exceeds a certain level. A switch is thrown to restore circuit ...
... and bends if current exceeds a certain level. A switch is thrown to restore circuit ...
NM Strand
... 49. If 40.0 g of NaOH is dissolved in 200.g of water, what is the concentration? 50. A student spills a chemical in the laboratory. What should he do first? 51. A sour candy has a pH of: 52. A characteristic that can be observed or measured without changing the sample’s composition is 53. An experim ...
... 49. If 40.0 g of NaOH is dissolved in 200.g of water, what is the concentration? 50. A student spills a chemical in the laboratory. What should he do first? 51. A sour candy has a pH of: 52. A characteristic that can be observed or measured without changing the sample’s composition is 53. An experim ...
Exam 1 - UNC Physics and Astronomy
... 1. The force increases. 2. The force decreases. 3. The force remains constant. 7. An electron is released between the plates of a parallel-plate capacitor. What can be said about the electrical potential of the electron and its electrical potential energy as it moves between the plates? 1. Both its ...
... 1. The force increases. 2. The force decreases. 3. The force remains constant. 7. An electron is released between the plates of a parallel-plate capacitor. What can be said about the electrical potential of the electron and its electrical potential energy as it moves between the plates? 1. Both its ...
Portable Appliance Testing Course Pre-Study Revision
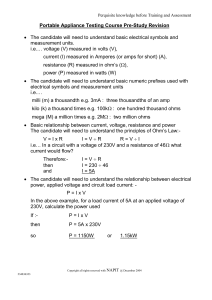
... The candidate will need to understand basic electrical symbols and measurement units. i.e.… voltage (V) measured in volts (V), current (I) measured in Amperes (or amps for short) (A), resistance (R) measured in ohm’s (), power (P) measured in watts (W) The candidate will need to understand basi ...
... The candidate will need to understand basic electrical symbols and measurement units. i.e.… voltage (V) measured in volts (V), current (I) measured in Amperes (or amps for short) (A), resistance (R) measured in ohm’s (), power (P) measured in watts (W) The candidate will need to understand basi ...
form revision a
... There are two types of compound. Covalent compounds form when non-metal atoms form covalent bonds by sharing their outer electrons. Covalent compounds exist as molecules. Ionic compounds form when metal atoms join to non-metal atoms by transferring electron(s) from the metal to the non-metal. The re ...
... There are two types of compound. Covalent compounds form when non-metal atoms form covalent bonds by sharing their outer electrons. Covalent compounds exist as molecules. Ionic compounds form when metal atoms join to non-metal atoms by transferring electron(s) from the metal to the non-metal. The re ...
Chapter 7 Notes
... In most atoms, the negative charge cancels out the positive charge. These atoms are said to be electrically neutral. ...
... In most atoms, the negative charge cancels out the positive charge. These atoms are said to be electrically neutral. ...
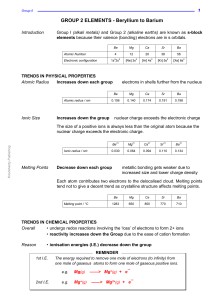
GROUP 2 ELEMENTS - Beryllium to Barium
... the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentration of OH¯ ions in water ...
... the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentration of OH¯ ions in water ...
General Chemistry - Review for final exam: (Make sure you bring
... 71. In the above reaction, NaCl + F2 NaF + Cl2, F is more or less reactive than Cl? 72. In the activity series of metals are the more reactive metals found on the top or the bottom of the chart? 73. What conditions in the reactants must be present in order for a double-replacement to take place? 7 ...
... 71. In the above reaction, NaCl + F2 NaF + Cl2, F is more or less reactive than Cl? 72. In the activity series of metals are the more reactive metals found on the top or the bottom of the chart? 73. What conditions in the reactants must be present in order for a double-replacement to take place? 7 ...
Chapter 7 Part1
... Electric current is a flow of electrons. In a circuit, electrons (negatively charged) actually flow through the metal wires. Conventional electric current is defined using the flow of positive charges. It is customary to use a conventional current I in the opposite direction to the electron flow. ...
... Electric current is a flow of electrons. In a circuit, electrons (negatively charged) actually flow through the metal wires. Conventional electric current is defined using the flow of positive charges. It is customary to use a conventional current I in the opposite direction to the electron flow. ...
ACA__Beat_sheet_bonding_2016
... BEAT Chemistry! (BE Able To): Bonding Determine if the following are ionic or covalent compounds A. NH3 = C. SiCl4= B. AlP = ...
... BEAT Chemistry! (BE Able To): Bonding Determine if the following are ionic or covalent compounds A. NH3 = C. SiCl4= B. AlP = ...
Nanofluidic circuitry
Nanofluidic circuitry is a nanotechnology aiming for control of fluids in nanometer scale. Due to the effect of an electrical double layer within the fluid channel, the behavior of nanofluid is observed to be significantly different compared with its microfluidic counterparts. Its typical characteristic dimensions fall within the range of 1–100 nm. At least one dimension of the structure is in nanoscopic scale. Phenomena of fluids in nano-scale structure are discovered to be of different properties in electrochemistry and fluid dynamics.