Unit 5 • What Do Atoms Look Like
... reaction. NH3 (“have pair will share”) is the Lewis base. The bond formed is a coordinate covalent bond with the shared pair of electrons being supplied solely by the nitrogen atom. Ksp of Ca(OH)2 Lab: In the Sally Ann Vonderbrink lab book, there is an elegant little lab to find the Ksp of Ca(OH)2. ...
... reaction. NH3 (“have pair will share”) is the Lewis base. The bond formed is a coordinate covalent bond with the shared pair of electrons being supplied solely by the nitrogen atom. Ksp of Ca(OH)2 Lab: In the Sally Ann Vonderbrink lab book, there is an elegant little lab to find the Ksp of Ca(OH)2. ...
L6452
... Print Head Block Diagram (Figure 5.) At first we have a constant current source, which can be disabled by an external pin (OnEnable) or by a control register, described later. The value of the current can be programmed by an external resistor, and is given by: V ref ⋅ 4 I CCS = --------------------2 ...
... Print Head Block Diagram (Figure 5.) At first we have a constant current source, which can be disabled by an external pin (OnEnable) or by a control register, described later. The value of the current can be programmed by an external resistor, and is given by: V ref ⋅ 4 I CCS = --------------------2 ...
Semiconductor device fabrication
... (CMP) is also a removal process used between levels. Patterning covers the series of processes that shape or alter the existing shape of the deposited materials and is generally referred to as lithography. For example, in conventional lithography, the wafer is coated with a chemical called a “photor ...
... (CMP) is also a removal process used between levels. Patterning covers the series of processes that shape or alter the existing shape of the deposited materials and is generally referred to as lithography. For example, in conventional lithography, the wafer is coated with a chemical called a “photor ...
3.10 Neutralization
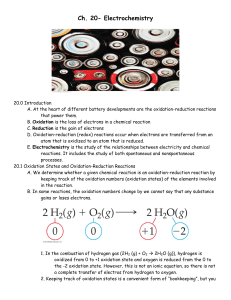
... • Transfer of electrons from one species to another 2Na(s) + Cl2(g) → 2NaCl(s) NaCl(s) consists of ions: 2Na(s) + Cl2(g) → 2Na+(s) + 2Cl-(s) Na(s) → Na+(s) ⇒ loss of 1e- by Na Cl2(g) → 2Cl-(s) ⇒ gain of 2e- by Cl2 Result: transfer of electrons from Na to Cl2 ...
... • Transfer of electrons from one species to another 2Na(s) + Cl2(g) → 2NaCl(s) NaCl(s) consists of ions: 2Na(s) + Cl2(g) → 2Na+(s) + 2Cl-(s) Na(s) → Na+(s) ⇒ loss of 1e- by Na Cl2(g) → 2Cl-(s) ⇒ gain of 2e- by Cl2 Result: transfer of electrons from Na to Cl2 ...
Document
... Atoms that share electrons can also form giant structures or macromolecules. Diamond and graphite (forms of carbon) and silicon dioxide (silica) are examples of giant covalent structures (lattices) of atoms. All the atoms in these structures are linked to other atoms by strong covalent bonds and so ...
... Atoms that share electrons can also form giant structures or macromolecules. Diamond and graphite (forms of carbon) and silicon dioxide (silica) are examples of giant covalent structures (lattices) of atoms. All the atoms in these structures are linked to other atoms by strong covalent bonds and so ...
Electronic Engineering
... components, which responds in the same way as the nonlinear active device. The equivalent circuit may not be perfect but will often give us a starting point when designing. Note that electronics on the whole is far from exact as we are working with components with relatively high tolerance: Resistor ...
... components, which responds in the same way as the nonlinear active device. The equivalent circuit may not be perfect but will often give us a starting point when designing. Note that electronics on the whole is far from exact as we are working with components with relatively high tolerance: Resistor ...
Chemistry A - Montgomery County Public Schools
... transpose word equations into symbolic chemical equations and vice versa. use the activity series to determine if single displacement reactions will occur. use solubility rules to predict if a precipitate will form in a double displacement reaction. use coefficients to balance simple chemica ...
... transpose word equations into symbolic chemical equations and vice versa. use the activity series to determine if single displacement reactions will occur. use solubility rules to predict if a precipitate will form in a double displacement reaction. use coefficients to balance simple chemica ...
Second-Order Circuits (7.3)
... The Differential Equation Most circuits with one capacitor and inductor are not as easy to analyze as the previous circuit. However, every voltage and current in such a circuit is the solution to a differential equation of the following form: ...
... The Differential Equation Most circuits with one capacitor and inductor are not as easy to analyze as the previous circuit. However, every voltage and current in such a circuit is the solution to a differential equation of the following form: ...
devices to protect against electrical hazards
... protects against a shock that occurs if person touches H with one hand and G with the other GFI opens power lead if H lead current differs more than 2 mA from N lead current for duration longer than 2 ms. ...
... protects against a shock that occurs if person touches H with one hand and G with the other GFI opens power lead if H lead current differs more than 2 mA from N lead current for duration longer than 2 ms. ...
Word - chemmybear.com
... electrode (where K+ is attracted) and have it react with water to form H2 and OH-. K+ + e- K 2K° + 2H2O 2K+ + 2OH- + H2 The combination of these two reactions is exactly what happens when water is reduced at the cathode. 8. (Trick #2) When CuSO4(aq) is electrolyzed, you know that Cu° metal is go ...
... electrode (where K+ is attracted) and have it react with water to form H2 and OH-. K+ + e- K 2K° + 2H2O 2K+ + 2OH- + H2 The combination of these two reactions is exactly what happens when water is reduced at the cathode. 8. (Trick #2) When CuSO4(aq) is electrolyzed, you know that Cu° metal is go ...
Nanofluidic circuitry
Nanofluidic circuitry is a nanotechnology aiming for control of fluids in nanometer scale. Due to the effect of an electrical double layer within the fluid channel, the behavior of nanofluid is observed to be significantly different compared with its microfluidic counterparts. Its typical characteristic dimensions fall within the range of 1–100 nm. At least one dimension of the structure is in nanoscopic scale. Phenomena of fluids in nano-scale structure are discovered to be of different properties in electrochemistry and fluid dynamics.