Protein Purification and Analysis
... (2) Choose source (natural or expressed) Break open cells by destroying membranes and releasing cytosolic protein mix - crude extract If nuclear or membrane protein - more work! (3) Soluble in aqueous solution?? (problem with membrane proteins) (4) Stability (perform purification/analyses in cold) ( ...
... (2) Choose source (natural or expressed) Break open cells by destroying membranes and releasing cytosolic protein mix - crude extract If nuclear or membrane protein - more work! (3) Soluble in aqueous solution?? (problem with membrane proteins) (4) Stability (perform purification/analyses in cold) ( ...
No Slide Title
... (2) Choose source (natural or expressed) Break open cells by destroying membranes and releasing cytosolic protein mix - crude extract If nuclear or membrane protein - more work! (3) Soluble in aqueous solution?? (problem with membrane proteins) (4) Stability (perform purification/analyses in cold) ( ...
... (2) Choose source (natural or expressed) Break open cells by destroying membranes and releasing cytosolic protein mix - crude extract If nuclear or membrane protein - more work! (3) Soluble in aqueous solution?? (problem with membrane proteins) (4) Stability (perform purification/analyses in cold) ( ...
Polymers - chemical engineering 2012
... •Propagation involves the linear growth of the polymer chain by the sequential addition of monomer units to this active growing chain molecule ...
... •Propagation involves the linear growth of the polymer chain by the sequential addition of monomer units to this active growing chain molecule ...
AP Chemistry Unit 1 Essential Questions Screencast 1
... 3. When converting units with a power what is important to remember? Screencast 1-7 Basic Atomic Structure, Isotopes and Mass Spectroscopy 1. What are the 3 subatomic particles and where are they located inside the atom? 2. What are the relative masses of the 3 particles? 3. What is the atomic mass? ...
... 3. When converting units with a power what is important to remember? Screencast 1-7 Basic Atomic Structure, Isotopes and Mass Spectroscopy 1. What are the 3 subatomic particles and where are they located inside the atom? 2. What are the relative masses of the 3 particles? 3. What is the atomic mass? ...
Performa® DTR Gel Filtration Cartridges
... The drier the packing (longer centrifugation times and/or higher g forces), the longer it takes to recover product and the lower the overall recovery. Conversely, shorter spin times and lower speeds result in elution volumes higher than the input sample volume. See “Notes” for determination of RPM f ...
... The drier the packing (longer centrifugation times and/or higher g forces), the longer it takes to recover product and the lower the overall recovery. Conversely, shorter spin times and lower speeds result in elution volumes higher than the input sample volume. See “Notes” for determination of RPM f ...
Chapter 12 Chemical Quantities
... Chemical formula which contains the lowest whole number ratio of atoms Ionic formulas always contain the lowest whole number ratios Covalent compounds do not always contain the lowest whole number ratios ...
... Chemical formula which contains the lowest whole number ratio of atoms Ionic formulas always contain the lowest whole number ratios Covalent compounds do not always contain the lowest whole number ratios ...
Name
... most carbons. Be aware that organic chemists use many shortcuts in drawing complex molecules. They often do not include the letter C for carbon in ring structures. How many cards did you find? ______________ 18. What are the two forms of sugars? _____________________________________ 19. Which of the ...
... most carbons. Be aware that organic chemists use many shortcuts in drawing complex molecules. They often do not include the letter C for carbon in ring structures. How many cards did you find? ______________ 18. What are the two forms of sugars? _____________________________________ 19. Which of the ...
Linear Polymer
... Based on Thermal Processing Behaviour i) Thermoplastic Polymers (ii) Thermosetting Polymer ...
... Based on Thermal Processing Behaviour i) Thermoplastic Polymers (ii) Thermosetting Polymer ...
Chapter 12 Stoichiometry - Conejo Valley Unified School
... • Stoichiometry is the part of chemistry that studies amounts of reactants and products that are involved in reactions. ...
... • Stoichiometry is the part of chemistry that studies amounts of reactants and products that are involved in reactions. ...
MISE - Physical Basis of Chemistry
... The reference atom (isotope) was a particular isotope of carbon (C), i.e., “carbon-12”. It was symbolized as: 12C. The mass of one atom of this particular carbon atom was defined as exactly 12.0000…. atomic mass units (amu). So, the conversion factor is: 1 atom of 12C = 12.00… amu. This means that t ...
... The reference atom (isotope) was a particular isotope of carbon (C), i.e., “carbon-12”. It was symbolized as: 12C. The mass of one atom of this particular carbon atom was defined as exactly 12.0000…. atomic mass units (amu). So, the conversion factor is: 1 atom of 12C = 12.00… amu. This means that t ...
NCW 2008 – Having a Ball with Chemistry
... - Depending on if the students hold the pipet straight up versus at an angle, they can ‘stack’ fewer or more droplets on the penny because the size of the droplet varies depending on how they hold the pipet. If they hold the pipet straight when they drop the pure water, then they should also hold it ...
... - Depending on if the students hold the pipet straight up versus at an angle, they can ‘stack’ fewer or more droplets on the penny because the size of the droplet varies depending on how they hold the pipet. If they hold the pipet straight when they drop the pure water, then they should also hold it ...
Cell Respiration Flow Chart
... Step 5.T he final process of cellular respiration takes place on the inner membrane of the mitochondria. This inner membrane is much larger than the mitochondria’s outer membrane. In mitochondria that are in the liver, the inner membrane is nearly five times the area of the outer membrane. In mit ...
... Step 5.T he final process of cellular respiration takes place on the inner membrane of the mitochondria. This inner membrane is much larger than the mitochondria’s outer membrane. In mitochondria that are in the liver, the inner membrane is nearly five times the area of the outer membrane. In mit ...
Compulsory textbook Recommended textbooks Topics of the first
... represents the object to be analysed ...
... represents the object to be analysed ...
here
... homology vs analogy A priori sequences could be similar due to convergent evolution Homology (shared ancestry) versus Analogy (convergent evolution) ...
... homology vs analogy A priori sequences could be similar due to convergent evolution Homology (shared ancestry) versus Analogy (convergent evolution) ...
Chapter 13…States of Matter
... 4. Calculate the amount of energy required to heat a 150 g chunk of aluminum from 20C to 40C. (Cp of aluminum = 0.220 cal/gC) H=mCpT (150g)(.22)(20) = 660 cal Chapters 17& 18…Reaction Rates & Equilibrium Define: 1. Equilibrium: the reaction occurs simultaneously in both directions. 2. Activate ...
... 4. Calculate the amount of energy required to heat a 150 g chunk of aluminum from 20C to 40C. (Cp of aluminum = 0.220 cal/gC) H=mCpT (150g)(.22)(20) = 660 cal Chapters 17& 18…Reaction Rates & Equilibrium Define: 1. Equilibrium: the reaction occurs simultaneously in both directions. 2. Activate ...
Chemistry of Metabolism
... energy. We can never detect energy directly. We only study energy through its effects on matter. Matter is composed of atoms. An atom is the smallest particles into which an element can be divided and still have the properties of that element. There are many different types of atoms called elements. ...
... energy. We can never detect energy directly. We only study energy through its effects on matter. Matter is composed of atoms. An atom is the smallest particles into which an element can be divided and still have the properties of that element. There are many different types of atoms called elements. ...
Chapter 3. Stoichiometry
... Convert grams of reactant to moles of reactant (use molar mass), Convert moles of one reactant to moles of other reactants and products (use the stoichiometric ratio from the balanced chemical equation), Convert moles back into grams for desired product (use molar mass). ...
... Convert grams of reactant to moles of reactant (use molar mass), Convert moles of one reactant to moles of other reactants and products (use the stoichiometric ratio from the balanced chemical equation), Convert moles back into grams for desired product (use molar mass). ...
Chapter 3 - Bruder Chemistry
... Balancing equations requires some trial and error. Algorithm loving students find this uncomfortable. Some students cannot distinguish between the number of moles actually manipulated in the laboratory versus the number of moles required by stoichiometry. Students do not appreciate that the coeffici ...
... Balancing equations requires some trial and error. Algorithm loving students find this uncomfortable. Some students cannot distinguish between the number of moles actually manipulated in the laboratory versus the number of moles required by stoichiometry. Students do not appreciate that the coeffici ...
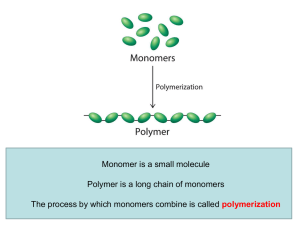
2.3 Carbon-Based Molecules
... • Many carbon-based molecules are made of many small subunits bonded together. – Monomers are the individual subunits. – Polymers are made of many monomers. ...
... • Many carbon-based molecules are made of many small subunits bonded together. – Monomers are the individual subunits. – Polymers are made of many monomers. ...
Honors-Final-Review-2014
... a. 101.3 kPa, zero degrees Celsius _____ 2. STP b. All matter composed of atoms/molecules, particles move in random motion, elastic collisions _____ 3. Boyle’s Law c. Gas escaping through a tiny hole _____ 4. Charles Law d. Volume inversely proportional to pressure _____ 5. Gay-Lussac’s Law e. Volum ...
... a. 101.3 kPa, zero degrees Celsius _____ 2. STP b. All matter composed of atoms/molecules, particles move in random motion, elastic collisions _____ 3. Boyle’s Law c. Gas escaping through a tiny hole _____ 4. Charles Law d. Volume inversely proportional to pressure _____ 5. Gay-Lussac’s Law e. Volum ...
Tutorial 3 (Ans Scheme) ERT 317, Sem 1 2015/2016
... Briggs and Haldane first proposed Quasi-steady-state assumption ...
... Briggs and Haldane first proposed Quasi-steady-state assumption ...
The Breakdown of Glucose (aka Cellular Respiration)
... Directions for making your FLIP BOOK 1. You will need at least 20 index cards (3x5 okay but 5x7 is better) or ½ sheets of card stock. 2. Hole punch a corner (or two places along the top) and attach a ring or string. 3. Your first card will be a TITLE card: “Breaking Down Glucose”; add your name and ...
... Directions for making your FLIP BOOK 1. You will need at least 20 index cards (3x5 okay but 5x7 is better) or ½ sheets of card stock. 2. Hole punch a corner (or two places along the top) and attach a ring or string. 3. Your first card will be a TITLE card: “Breaking Down Glucose”; add your name and ...
Cellular Respiration Lecture Notes
... products during the first 2 stages 3. Passes electrons from one molecule to another 4. electrons combined with hydrogen ions 5. molecular oxygen to form water 6. energy released at each step of the chain is stored in mitochondria to make ATP ii. Substrate level phosphorylation 1. Forms smaller amoun ...
... products during the first 2 stages 3. Passes electrons from one molecule to another 4. electrons combined with hydrogen ions 5. molecular oxygen to form water 6. energy released at each step of the chain is stored in mitochondria to make ATP ii. Substrate level phosphorylation 1. Forms smaller amoun ...
Size-exclusion chromatography

Size-exclusion chromatography (SEC) is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.