Chem A Week 2 Matter Notes
... contact remain in __________ with each other at all times as the slide around, so there is not much energy change in __________. The forces are strong enough to keep the particles from flying away. ...
... contact remain in __________ with each other at all times as the slide around, so there is not much energy change in __________. The forces are strong enough to keep the particles from flying away. ...
homework assignment 2 - the Petersen Home Page
... 1. A 15.40-g sample of a finely-divided mixture of only Fe2S3 and FeS was reacted with excess H2 at elevated temperatures. If the weight percent of Fe2S3 in this mixture is 57.4%, then calculate the total mass in grams of Fe that can be produced. Assume the only other product of these reactions is H ...
... 1. A 15.40-g sample of a finely-divided mixture of only Fe2S3 and FeS was reacted with excess H2 at elevated temperatures. If the weight percent of Fe2S3 in this mixture is 57.4%, then calculate the total mass in grams of Fe that can be produced. Assume the only other product of these reactions is H ...
Chem 1151: Ch. 2 - Clayton State University
... • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of this isotope notation is magnesium-26. This represents an isotope of magnesium that has a mass number of 26. ...
... • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of this isotope notation is magnesium-26. This represents an isotope of magnesium that has a mass number of 26. ...
I Examen I Trim Science
... A solid is the state of matter that has a definite shape and volume. The particles in a solid do not move fast enough to overcome the attraction between them. Each particle vibrates in place and is locked in place by the particles around it. 2 types of solids: Crystalline solids: have a very ...
... A solid is the state of matter that has a definite shape and volume. The particles in a solid do not move fast enough to overcome the attraction between them. Each particle vibrates in place and is locked in place by the particles around it. 2 types of solids: Crystalline solids: have a very ...
analysis of membrane protein dimerization
... to significantly and completely (respectively) reduce dimerization, as measured by SDS-PAGE. K 2,1 estimates of 1.4 (±0.2) and 4.2 (±0.9) µM were obtained for these mutants, respectively, using sedimentation equilibrium in C8E5 at 25°C. The differences in the free energies between the wild type and ...
... to significantly and completely (respectively) reduce dimerization, as measured by SDS-PAGE. K 2,1 estimates of 1.4 (±0.2) and 4.2 (±0.9) µM were obtained for these mutants, respectively, using sedimentation equilibrium in C8E5 at 25°C. The differences in the free energies between the wild type and ...
eprint_5_24935_775
... Polymers are long chain molecules with a wide range of physical and chemical properties. One of the main advantages of the polymer materials is the ease of fabrication to produce various shapes (rod, film, fiber, sheet, etc.). The advances in polymer chemistry have made it possible to tailor the pro ...
... Polymers are long chain molecules with a wide range of physical and chemical properties. One of the main advantages of the polymer materials is the ease of fabrication to produce various shapes (rod, film, fiber, sheet, etc.). The advances in polymer chemistry have made it possible to tailor the pro ...
Chemistry 2000 (Fall 2008) Problem Set #4: Intermolecular Forces
... forces present are induced dipole-induced dipole forces • CCl4 is smaller than CI4. Therefore, CI4 has a much higher polarizibility and has much stronger induced dipole-induced dipole forces. As a consequence, CI4 has a lower vapour pressure and a higher boiling point than CCl4. • The difference in ...
... forces present are induced dipole-induced dipole forces • CCl4 is smaller than CI4. Therefore, CI4 has a much higher polarizibility and has much stronger induced dipole-induced dipole forces. As a consequence, CI4 has a lower vapour pressure and a higher boiling point than CCl4. • The difference in ...
8 M Guanidine Hydrochloride Solution Buffered, pH - Sigma
... Ammonium Bicarbonate Solution for 1 hour at room temperature. 6. Replace the dialysis buffer with fresh 100 mM Ammonium Bicarbonate Solution and continue to dialyze for another 2 hours. If a precipitate forms during dialysis, resuspend the precipitated proteins by pipetting the solution up and down. ...
... Ammonium Bicarbonate Solution for 1 hour at room temperature. 6. Replace the dialysis buffer with fresh 100 mM Ammonium Bicarbonate Solution and continue to dialyze for another 2 hours. If a precipitate forms during dialysis, resuspend the precipitated proteins by pipetting the solution up and down. ...
Chapter 3
... By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). The molar mass of an element is the mass number for the element that we find on the periodic table. The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). ...
... By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). The molar mass of an element is the mass number for the element that we find on the periodic table. The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). ...
Bioinorganic Chemistry of Metal
... variety of functions including oxygen storage/transport, elec tron transfer, redox catalysis with various substrates. Besides these traditional functions of hemeproteins, a new function of hemeprotein has been found recently, which is a sensor of diatomic gas molecules or redox change. 1) In these ...
... variety of functions including oxygen storage/transport, elec tron transfer, redox catalysis with various substrates. Besides these traditional functions of hemeproteins, a new function of hemeprotein has been found recently, which is a sensor of diatomic gas molecules or redox change. 1) In these ...
E. coli
... • The size of a bacterial cell is around 1 µm with a weight of 1 pg. • The interior of the cell is a viscous solution crowded with several molecular species • The cells are mostly composed of water and macromolecules with simple metabolites forming only a small fraction. • Typical concentrations of ...
... • The size of a bacterial cell is around 1 µm with a weight of 1 pg. • The interior of the cell is a viscous solution crowded with several molecular species • The cells are mostly composed of water and macromolecules with simple metabolites forming only a small fraction. • Typical concentrations of ...
Chem 1A Practice Final
... 7. A solution is prepared by dissolving 0.115 moles of ammonium sulfate, (NH4)2SO4, in enough water to make 100.0 mL of stock solution. A 11.00 mL sample of this stock solution is added to 50.00 mL of water. Calculate the concentration of ammonium ions in the final solution. a) 1.15 M b) 0.51 M c) ...
... 7. A solution is prepared by dissolving 0.115 moles of ammonium sulfate, (NH4)2SO4, in enough water to make 100.0 mL of stock solution. A 11.00 mL sample of this stock solution is added to 50.00 mL of water. Calculate the concentration of ammonium ions in the final solution. a) 1.15 M b) 0.51 M c) ...
4) Protein Evolution
... Principle: Use the unique physicochemical properties of your protein to separate it from all the others ...
... Principle: Use the unique physicochemical properties of your protein to separate it from all the others ...
Reactive Oxygen Species Scavenging Activity of Flavone Glycosides from Melilotus neapolitana
... region of the 1H-NMR spectrum an AA'BB' system, appearing as two doublets at δ 8.12 and 6.89 and two meta coupled doublet protons at δ 6.75 and 6.51 were evident. In the saccharide region of the spectrum three anomeric proton signals were present, indicating the presence of three sugar moieties. Two ...
... region of the 1H-NMR spectrum an AA'BB' system, appearing as two doublets at δ 8.12 and 6.89 and two meta coupled doublet protons at δ 6.75 and 6.51 were evident. In the saccharide region of the spectrum three anomeric proton signals were present, indicating the presence of three sugar moieties. Two ...
Rapid purification of heart muscle enzymes using dye affinity
... the ammonium sulphate fractionation. The affinity aqueous two-phase systems resulted in good recovery of both LDH and PK from the ammonium sulphate fraction, with purification factors that are consistent with conventional dye affinity column chromatography [3]. Previous workers have demonstrated dif ...
... the ammonium sulphate fractionation. The affinity aqueous two-phase systems resulted in good recovery of both LDH and PK from the ammonium sulphate fraction, with purification factors that are consistent with conventional dye affinity column chromatography [3]. Previous workers have demonstrated dif ...
STOICHIOMETRY (I) Molecular Mass: The sum of the masses of the
... The reactant that is completely consumed in a chemical reaction and that limits the amount of product formed. Calculate the expected yield using each of the given reactant amounts. Whichever reactant gives the least product is the limiting reactant. Ex: a) Iron (II) sulfide reacts with hydrochloric ...
... The reactant that is completely consumed in a chemical reaction and that limits the amount of product formed. Calculate the expected yield using each of the given reactant amounts. Whichever reactant gives the least product is the limiting reactant. Ex: a) Iron (II) sulfide reacts with hydrochloric ...
Chapter 5 - Scranton Prep Biology
... acids can severely affect a protein's function by altering the protein's conformation. A substitution of only one of the 145amino acids in the primary structure of hemoglobin causessickle-celldisease. The interactions that createand maintain secondary and tertiary structure can be disrupted by chang ...
... acids can severely affect a protein's function by altering the protein's conformation. A substitution of only one of the 145amino acids in the primary structure of hemoglobin causessickle-celldisease. The interactions that createand maintain secondary and tertiary structure can be disrupted by chang ...
CHEMISTRY - careerpoint.ac.in
... side must be equal to the total number of atoms of the same element on the products side. This law will be applied in balancing the chemical equation. ...
... side must be equal to the total number of atoms of the same element on the products side. This law will be applied in balancing the chemical equation. ...
homework_#1_10
... of atoms on each side and the same total mass on each side. You DO NOT have the same number of MOLES on each side (7 on the left, 6 on the right) or VOLUME (7 x 22.4 Liters on the left, 6 x 22.4 on the right) or MOLECULES (7 on the left, 6 on the right) ...
... of atoms on each side and the same total mass on each side. You DO NOT have the same number of MOLES on each side (7 on the left, 6 on the right) or VOLUME (7 x 22.4 Liters on the left, 6 x 22.4 on the right) or MOLECULES (7 on the left, 6 on the right) ...
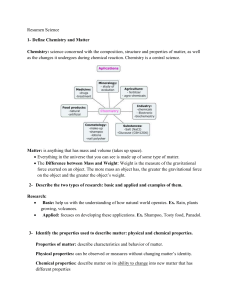
Resumen Science I Trimestre II Parcial Definitions: Element: pure
... Molecule: particle of a compound; formed when atoms of 2 or more elements join together. Compound: pure substances composed of 2 or more elements that are chemically combined; elements combine reaction with one another. Chemical Formula: is the fundamental unit of an element. The symbols for the ele ...
... Molecule: particle of a compound; formed when atoms of 2 or more elements join together. Compound: pure substances composed of 2 or more elements that are chemically combined; elements combine reaction with one another. Chemical Formula: is the fundamental unit of an element. The symbols for the ele ...
Polymer Lesson - Penn Arts and Sciences
... recessive form of the gene became much more common and more and more people inherited the disease. Sickle cell anemia is caused by a genetic change in hemoglobin. Hemoglobin is a protein that is made up of chains of amino acids. Its function is to pick up oxygen in our lungs and deliver it to other ...
... recessive form of the gene became much more common and more and more people inherited the disease. Sickle cell anemia is caused by a genetic change in hemoglobin. Hemoglobin is a protein that is made up of chains of amino acids. Its function is to pick up oxygen in our lungs and deliver it to other ...
Fast Separation of Recombinant Human Erythropoietin
... 60 °C at different pH. At neutral pH, rEPO forms limited isoforms, but when acidic pH conditions are used, the structure of rEPO protein will be altered significantly. The lower the pH, the more changes1. Figure 3 shows both conditions of heating. Panel A shows data from a sample was heated at neutr ...
... 60 °C at different pH. At neutral pH, rEPO forms limited isoforms, but when acidic pH conditions are used, the structure of rEPO protein will be altered significantly. The lower the pH, the more changes1. Figure 3 shows both conditions of heating. Panel A shows data from a sample was heated at neutr ...
The Additive Screen MD1-11
... minimum dilution of the sample drop, and are sufficiently concentrated that vapour diffusion experiments can be carried out in as little as a 2.5µl drop. All additives have to be added to the sample protein. Non-volatile reagents can be added directly to a sitting or hanging drop. Volatile reagents ...
... minimum dilution of the sample drop, and are sufficiently concentrated that vapour diffusion experiments can be carried out in as little as a 2.5µl drop. All additives have to be added to the sample protein. Non-volatile reagents can be added directly to a sitting or hanging drop. Volatile reagents ...
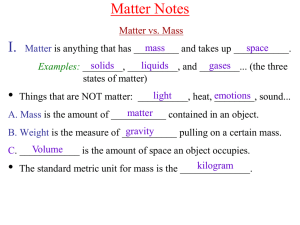
Matter - tompkinsmath
... Do not conduct electricity when dissolved in water. - non-metal atoms share electrons to achieve a stable state (instead of gaining or losing electrons.) - This “sharing” holds the atoms together is a group called a molecule. - A molecular formula indicates the number of each in a molecule. Ex. H2O ...
... Do not conduct electricity when dissolved in water. - non-metal atoms share electrons to achieve a stable state (instead of gaining or losing electrons.) - This “sharing” holds the atoms together is a group called a molecule. - A molecular formula indicates the number of each in a molecule. Ex. H2O ...
2004 NEACS Ashdown Exam 1. The allotrope of carbon shown to
... (A) When gas molecules collide, there is no loss in kinetic energy. (B) Gases have no volume. (C) All gases are moving with the same velocity at the same temperature. (D) The average kinetic energy of a gas is proportional to its temperature. 12. The solution with the lowest boiling point is (A) 0.0 ...
... (A) When gas molecules collide, there is no loss in kinetic energy. (B) Gases have no volume. (C) All gases are moving with the same velocity at the same temperature. (D) The average kinetic energy of a gas is proportional to its temperature. 12. The solution with the lowest boiling point is (A) 0.0 ...
Size-exclusion chromatography

Size-exclusion chromatography (SEC) is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.