Core organic chemistry
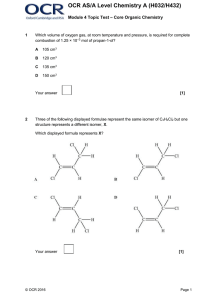
... of isomers linked to the reaction, with clear understanding of both types of isomerism The explanations show a well-developed line of reasoning which is clear and logically structured. The information presented is relevant to the compounds ...
... of isomers linked to the reaction, with clear understanding of both types of isomerism The explanations show a well-developed line of reasoning which is clear and logically structured. The information presented is relevant to the compounds ...
File - Junior College Chemistry tuition
... 15 The following two experiments are carried out with anhydrous potassium chloride and observations X and Y are made at the end of each experiment. Concentrated sulfuric acid is added to the potassium chloride and the fumes produced are bubbled into aqueous potassium bromide solution – observation X ...
... 15 The following two experiments are carried out with anhydrous potassium chloride and observations X and Y are made at the end of each experiment. Concentrated sulfuric acid is added to the potassium chloride and the fumes produced are bubbled into aqueous potassium bromide solution – observation X ...
Regents Chemistry Review - New York Science Teacher
... In the laboratory, a glass tube is filled with hydrogen gas at a very low pressure. When a scientist applies a high voltage between metal electrodes in the tube, light .is emitted. When the light is analyzed with a spectroscope four distinct spectral lines are noted. Information on their frequency ...
... In the laboratory, a glass tube is filled with hydrogen gas at a very low pressure. When a scientist applies a high voltage between metal electrodes in the tube, light .is emitted. When the light is analyzed with a spectroscope four distinct spectral lines are noted. Information on their frequency ...
Use the following answers for questions 1
... 30. Hydrogen gas is collected over water at 24 °C. The total pressure of the sample is 755 millimeters of mercury. At 24 °C, the vapor pressure of water is 22 millimeters of mercury. What is the partial pressure of the hydrogen gas? (A) 22 mm Hg (B) 733 mm Hg (C) 755 mm Hg (D) 760 mm Hg (E) 777 mm H ...
... 30. Hydrogen gas is collected over water at 24 °C. The total pressure of the sample is 755 millimeters of mercury. At 24 °C, the vapor pressure of water is 22 millimeters of mercury. What is the partial pressure of the hydrogen gas? (A) 22 mm Hg (B) 733 mm Hg (C) 755 mm Hg (D) 760 mm Hg (E) 777 mm H ...
Chapter 10
... ammonia from its elements is N2(g) + 3 H2(g) 2 NH3(g) Assuming all gases are at the same temperature and pressure, how many liters of ammonia are formed from 2.70 liters of hydrogen? ...
... ammonia from its elements is N2(g) + 3 H2(g) 2 NH3(g) Assuming all gases are at the same temperature and pressure, how many liters of ammonia are formed from 2.70 liters of hydrogen? ...
Equilibrium Notes - Chemistry Teaching Resources
... show no units but do not explain the reasons why. A very brief and simplistic explanation is given below. Equilibrium constants should be calculated using activity (relative concentration) not actual concentration. Simply, the concentration terms in the equilibrium constant equation shown above shou ...
... show no units but do not explain the reasons why. A very brief and simplistic explanation is given below. Equilibrium constants should be calculated using activity (relative concentration) not actual concentration. Simply, the concentration terms in the equilibrium constant equation shown above shou ...
PDF File
... with trace [γ-32P]ATP. In all reactions, ionic strength was maintained at 0.3 M (KCl). Reaction rates were linearly dependent upon ATP concentration under these conditions, indicating that ATP was subsaturating. The reactions were pH-independent between pH 7 and 9, suggesting that Glu 133, a residue ...
... with trace [γ-32P]ATP. In all reactions, ionic strength was maintained at 0.3 M (KCl). Reaction rates were linearly dependent upon ATP concentration under these conditions, indicating that ATP was subsaturating. The reactions were pH-independent between pH 7 and 9, suggesting that Glu 133, a residue ...
4U Chemistry Practice Exam - Coristines
... b. Amines are non-polar molecules. c. Amines always have a larger molecular weight than amides. d. Amines always have a nitrogen atom attached to two carbon atoms. e. Amines can be found in proteins, but amides can not. 5. Why does the boiling point of an alkane increase as its chain length increase ...
... b. Amines are non-polar molecules. c. Amines always have a larger molecular weight than amides. d. Amines always have a nitrogen atom attached to two carbon atoms. e. Amines can be found in proteins, but amides can not. 5. Why does the boiling point of an alkane increase as its chain length increase ...
The Free High School Science Texts
... money, go ahead, distribute our books far and wide - we DARE you! • Ever wanted to change your textbook? Of course you have! Go ahead, change ours, make your own version, get your friends together, rip it apart and put it back together the way you like it. That’s what we really want! • Copy, modify, ...
... money, go ahead, distribute our books far and wide - we DARE you! • Ever wanted to change your textbook? Of course you have! Go ahead, change ours, make your own version, get your friends together, rip it apart and put it back together the way you like it. That’s what we really want! • Copy, modify, ...
Abstract - Engineering | UMass
... becomes exhausted, TTHM formation nearly stops. The same general characteristics apply for chlorine demand (Figure 4). However, DCAN shows a different behavior ...
... becomes exhausted, TTHM formation nearly stops. The same general characteristics apply for chlorine demand (Figure 4). However, DCAN shows a different behavior ...
Ch 16 Power Point
... • The enthalpy change that occurs during the complete combustion of one mole of a substance is called the enthalpy of combustion of the substance. • Enthalpy of combustion is defined in terms of one mole of reactant, whereas the enthalpy of formation is defined in terms of one mole of product. • ∆H ...
... • The enthalpy change that occurs during the complete combustion of one mole of a substance is called the enthalpy of combustion of the substance. • Enthalpy of combustion is defined in terms of one mole of reactant, whereas the enthalpy of formation is defined in terms of one mole of product. • ∆H ...
Fill in the blanks with the appropriate word, phrase, number, or unit.
... 2. (3 points) The molecular formula of a compound with the empirical formula H5C2O and a molecular mass of 90.0 g/mole is ...
... 2. (3 points) The molecular formula of a compound with the empirical formula H5C2O and a molecular mass of 90.0 g/mole is ...