Section 2 Oxidation Numbers
... • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, _________ ________ are assigned to the atoms composing the compound or ion. • Unlike ionic charges, _______ ________ do not have an ______ ________ meaning: rather, ...
... • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, _________ ________ are assigned to the atoms composing the compound or ion. • Unlike ionic charges, _______ ________ do not have an ______ ________ meaning: rather, ...
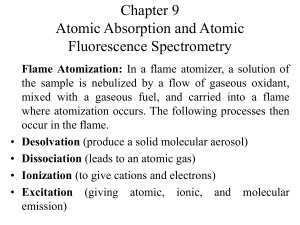
Chapter 9 Atomic Absorption and Atomic Fluorescence Spectrometry
... sample is atomized in a short period, and the average residence time of the atoms in the optical path is a second or more. A few microliters of sample are first evaporated at a low temperature and then ashed at a somewhat higher temperature in an electrically heated graphite tube or in a graphite cu ...
... sample is atomized in a short period, and the average residence time of the atoms in the optical path is a second or more. A few microliters of sample are first evaporated at a low temperature and then ashed at a somewhat higher temperature in an electrically heated graphite tube or in a graphite cu ...
Description: This is an advanced placement course designed to
... The following list of topics for an AP course is intended to be a guide to the level and breadth of treatment expected rather than to be a syllabus. The percentage after each major topic indicates the approximate proportion of multiple-choice questions on the examination that pertain to the topic. I ...
... The following list of topics for an AP course is intended to be a guide to the level and breadth of treatment expected rather than to be a syllabus. The percentage after each major topic indicates the approximate proportion of multiple-choice questions on the examination that pertain to the topic. I ...
Q - PIMS
... Ionization chamber: In this chamber fast moving electrons are bombarded which knock out electrons from neutral atoms. Thus they are converted into ions. These particles may consist of single atoms having positive charge. They may have different masses depending upon the nature of isotopes of that el ...
... Ionization chamber: In this chamber fast moving electrons are bombarded which knock out electrons from neutral atoms. Thus they are converted into ions. These particles may consist of single atoms having positive charge. They may have different masses depending upon the nature of isotopes of that el ...
Document
... 55. An element with atomic number-26 is _____. A) Ca B) Fe C) Co D) Ni 56. The element [Ne]3s1 is in the _____ group. A) 1st B) 2nd C) 13th D) 17th 57. The element [Ne]3s23p3 is in the _____ group. A) 13th B) 2nd C) 15th D) 17th 58. The element [Ar]4s23d8 is a/an _____. A) alkali metal B) transition ...
... 55. An element with atomic number-26 is _____. A) Ca B) Fe C) Co D) Ni 56. The element [Ne]3s1 is in the _____ group. A) 1st B) 2nd C) 13th D) 17th 57. The element [Ne]3s23p3 is in the _____ group. A) 13th B) 2nd C) 15th D) 17th 58. The element [Ar]4s23d8 is a/an _____. A) alkali metal B) transition ...
Final Exam Review Packet
... 5. - The molecular weight is the sum of the atomic weights of the atoms in a molecule of a compound. - The formula weight is the sum of the atomic weights of the atoms in a formula unit. - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quanti ...
... 5. - The molecular weight is the sum of the atomic weights of the atoms in a molecule of a compound. - The formula weight is the sum of the atomic weights of the atoms in a formula unit. - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quanti ...
Preview Sample 1
... 13. The atomic ___________ of an atom of an element equals the number of protons and neutrons in its nucleus. ________________________________________ 14. The number of protons in the atoms of a particular element is called the element's atomic __________. ________________________________________ 1 ...
... 13. The atomic ___________ of an atom of an element equals the number of protons and neutrons in its nucleus. ________________________________________ 14. The number of protons in the atoms of a particular element is called the element's atomic __________. ________________________________________ 1 ...
6.02 × 1023 molecules = 1 mole
... One of the most well-known numbers in the study of chemistry is number of units in a mole. The number of units in a mole is called Avogadro’s number (named after the Italian physicist). The mole is defined as the number of atoms in 12.0 grams of 12C. As you can tell from the equality below, the mole ...
... One of the most well-known numbers in the study of chemistry is number of units in a mole. The number of units in a mole is called Avogadro’s number (named after the Italian physicist). The mole is defined as the number of atoms in 12.0 grams of 12C. As you can tell from the equality below, the mole ...
Module 9 Methods for Structure Determination Lecture 24 UV
... due to a similar fragmentation where one of the benzylic C-H is replaced by methyl group. Similarly, when an alkyl group attached to the benzene ring is a propyl group or larger, Mclafferty rearrangement occurs to give fragments. Using butyl benzene the effect of McLafferty rearrangement can be show ...
... due to a similar fragmentation where one of the benzylic C-H is replaced by methyl group. Similarly, when an alkyl group attached to the benzene ring is a propyl group or larger, Mclafferty rearrangement occurs to give fragments. Using butyl benzene the effect of McLafferty rearrangement can be show ...
AP Chemistry: Course Introduction Sheet
... Unit 2 -Atoms and Elements LEARNING TARGETS Parts of the Atom: ...
... Unit 2 -Atoms and Elements LEARNING TARGETS Parts of the Atom: ...
Stage 2 Chemistry Intended Student Learning 2014
... Topic 1: Elemental and Environmental Chemistry This topic deals with some of the underlying principles of chemistry (‘elemental chemistry’) and then considers the chemistry of the environment. The elemental chemistry component of the topic focuses on the periodic table and the concept of electroneg ...
... Topic 1: Elemental and Environmental Chemistry This topic deals with some of the underlying principles of chemistry (‘elemental chemistry’) and then considers the chemistry of the environment. The elemental chemistry component of the topic focuses on the periodic table and the concept of electroneg ...
GCE Getting Started - Edexcel
... © Pearson Education Ltd 2015. Copying permitted for purchasing institution only. This material is not copyright free. ...
... © Pearson Education Ltd 2015. Copying permitted for purchasing institution only. This material is not copyright free. ...
Exercise 4.1 – Masses of Particles Relative Isotopic Mass Chemists
... A ____________________________ is defined as the amount of substance that contains the same ____________________________ of specified particles as there are atoms in 12g of carbon-12. The mole has been defined in a convenient way: –1 atom of 12C has a relative ____________________________ mass of 12 ...
... A ____________________________ is defined as the amount of substance that contains the same ____________________________ of specified particles as there are atoms in 12g of carbon-12. The mole has been defined in a convenient way: –1 atom of 12C has a relative ____________________________ mass of 12 ...
Chem 2A Final Review
... 9. The answer closest to the number of grams of NaH2PO4 needed to react with 38.74 mL of 0.275 M NaOH, according to the following balanced equation is: (NaH2PO4 = 119.98 g/mol) NaH2PO4 (s) + 2NaOH (aq) Na3POH4 (aq) + 3H2O ...
... 9. The answer closest to the number of grams of NaH2PO4 needed to react with 38.74 mL of 0.275 M NaOH, according to the following balanced equation is: (NaH2PO4 = 119.98 g/mol) NaH2PO4 (s) + 2NaOH (aq) Na3POH4 (aq) + 3H2O ...
file - Mindset Learn
... In this series of six lessons, the learner will be exposed to the atomic model. The videos need to be viewed in order. By exploring the way the model was modified and rearranged as new and more accurate facts were discovered, learners will understand the nature of a scientific model. It all began ar ...
... In this series of six lessons, the learner will be exposed to the atomic model. The videos need to be viewed in order. By exploring the way the model was modified and rearranged as new and more accurate facts were discovered, learners will understand the nature of a scientific model. It all began ar ...
Chemistry Standards Clarification
... based on the melting point and boiling point of the substances. Compare the strength of the forces of attraction between molecules of different elements. (For example, at room temperature, chlorine is a gas and iodine is a solid.) Predict whether the forces of attraction in a solid are primarily met ...
... based on the melting point and boiling point of the substances. Compare the strength of the forces of attraction between molecules of different elements. (For example, at room temperature, chlorine is a gas and iodine is a solid.) Predict whether the forces of attraction in a solid are primarily met ...
Step 2
... charges, assigned using a set of rules, used to describe redox reactions with covalent compounds. ...
... charges, assigned using a set of rules, used to describe redox reactions with covalent compounds. ...
Chemical Quantities: Stoichiometry and the Mole
... you follow the steps you can get to the answer. Think about the “steps” I mentioned when we talked about problem solving. When we learned how to write the names of compounds for their formulas, and the formulas for the names. When we do conversions and stoichiometry. I am half cliche when I describe ...
... you follow the steps you can get to the answer. Think about the “steps” I mentioned when we talked about problem solving. When we learned how to write the names of compounds for their formulas, and the formulas for the names. When we do conversions and stoichiometry. I am half cliche when I describe ...
File
... (b) The protons and neutrons are located in a small nucleus at the centre of the atom. Due to the presence of protons, nucleus is positively charged. (c) The electrons revolve rapidly round the nucleus in fixed circular paths called energy levels or shells. The energy levels or shells are represente ...
... (b) The protons and neutrons are located in a small nucleus at the centre of the atom. Due to the presence of protons, nucleus is positively charged. (c) The electrons revolve rapidly round the nucleus in fixed circular paths called energy levels or shells. The energy levels or shells are represente ...
File
... Take time now to make sure your data table is complete for your Beanium activity we completed on Friday. If you did not get mass make sure to get that information from a classmate now. Check your calculations. Based on the lab activity answer the following question: How did the Beanium lab represent ...
... Take time now to make sure your data table is complete for your Beanium activity we completed on Friday. If you did not get mass make sure to get that information from a classmate now. Check your calculations. Based on the lab activity answer the following question: How did the Beanium lab represent ...
Document
... Stoichiometry: Calculations with Chemical Formulas and Equations Text: Sections E, F, and H ...
... Stoichiometry: Calculations with Chemical Formulas and Equations Text: Sections E, F, and H ...
Holt Modern Chemistry Workbook
... Electron-Dot Notation Usually, covalent bonds involve only the electrons in the outermost energy level of an atom. Electron-dot notation is useful for keeping track of these valence electrons. Electrondot notation is an electron-configuration notation in which only the valence electrons are shown, ...
... Electron-Dot Notation Usually, covalent bonds involve only the electrons in the outermost energy level of an atom. Electron-dot notation is useful for keeping track of these valence electrons. Electrondot notation is an electron-configuration notation in which only the valence electrons are shown, ...
Preview Sample 3
... with the electrons. 2. Page 33. Instructor’s Tip: If dimensional analysis did not identify the mathematically challenged students, calculating average atomic mass in this chapter will. Pointing out locations on campus where students can get help with their math skills may be helpful. 3. Page 33. Ins ...
... with the electrons. 2. Page 33. Instructor’s Tip: If dimensional analysis did not identify the mathematically challenged students, calculating average atomic mass in this chapter will. Pointing out locations on campus where students can get help with their math skills may be helpful. 3. Page 33. Ins ...
Introduction to Atomic Structure - New Jersey Center for Teaching
... Also remember that today we know atoms can be broken down into smaller bits. We also know all atoms of an element are not identical elements found in nature can vary in number of neutrons. However, for the purposes of general Chemistry, Dalton's Postulates are still a pretty reasonable approximation ...
... Also remember that today we know atoms can be broken down into smaller bits. We also know all atoms of an element are not identical elements found in nature can vary in number of neutrons. However, for the purposes of general Chemistry, Dalton's Postulates are still a pretty reasonable approximation ...
Chapter 3 - Robinson Schools
... contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound • Law of multiple proportions: if two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with ...
... contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound • Law of multiple proportions: if two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.