Moles Review
... 6) Given that exactly 4 quarts = 3.7854 liters convert the following: 2.85 quarts to liters and 7.888 liters to quarts 7) How many grams are there in 4.00 moles of He? 8) How many moles are there in 15.0 g of He? 9) How many grams are there in 0.025 mol of CO2? 10) How many moles are there in 4.02g ...
... 6) Given that exactly 4 quarts = 3.7854 liters convert the following: 2.85 quarts to liters and 7.888 liters to quarts 7) How many grams are there in 4.00 moles of He? 8) How many moles are there in 15.0 g of He? 9) How many grams are there in 0.025 mol of CO2? 10) How many moles are there in 4.02g ...
chemistry - My Study materials – Kumar
... All matters in the universe exist in three states. There are two ways of classification of matter. 1. According to physical state as solid, liquid or gas. 2. According to its composition as element, compound or mixture. According to this law mass of an isolated system will remain constant over time. ...
... All matters in the universe exist in three states. There are two ways of classification of matter. 1. According to physical state as solid, liquid or gas. 2. According to its composition as element, compound or mixture. According to this law mass of an isolated system will remain constant over time. ...
9.2 Oxidation Numbers
... The oxidation number of a monatomic ion is equal to its charge. When fluorine atoms are combined with atoms of other elements, their oxidation number is ‒1. When oxygen atoms are combined with atoms of other elements, their oxidation number is ‒2, except in peroxides, such as hydrogen peroxide, H2O2 ...
... The oxidation number of a monatomic ion is equal to its charge. When fluorine atoms are combined with atoms of other elements, their oxidation number is ‒1. When oxygen atoms are combined with atoms of other elements, their oxidation number is ‒2, except in peroxides, such as hydrogen peroxide, H2O2 ...
Atomic structure and periodic table
... There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table. A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Mosele ...
... There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table. A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Mosele ...
Chemistry Syllabus Grade 7
... Students should appreciate that chemistry is one of natural sciences and that this group also includes biology, geology and physics. Students could be asked to write one sentence about each natural science saying exactly what areas or aspects of nature are studied e.g. • Biology – study of living th ...
... Students should appreciate that chemistry is one of natural sciences and that this group also includes biology, geology and physics. Students could be asked to write one sentence about each natural science saying exactly what areas or aspects of nature are studied e.g. • Biology – study of living th ...
Unit 1 Atoms, Molecules and Stoichiometry
... The first chemist to use the name ‘atom’ was John Dalton (1766-1844). Dalton used the word ‘atom’ to mean the smallest particle of an element. He then went on to explain how atoms could react together to form molecules : An atom is the smallest part of an element which can ever exist. A molecule is ...
... The first chemist to use the name ‘atom’ was John Dalton (1766-1844). Dalton used the word ‘atom’ to mean the smallest particle of an element. He then went on to explain how atoms could react together to form molecules : An atom is the smallest part of an element which can ever exist. A molecule is ...
C H
... Configuration of a molecule – three-dimentional arrangement of atoms in the molecule. The ability to form two or more molecules with different configuration is called stereoisomerism. Stereocenter is defined as an atom bearing groups such that an interchanging of any two groups leads to a stereoisom ...
... Configuration of a molecule – three-dimentional arrangement of atoms in the molecule. The ability to form two or more molecules with different configuration is called stereoisomerism. Stereocenter is defined as an atom bearing groups such that an interchanging of any two groups leads to a stereoisom ...
STUDY MATERIAL 2015-16 CHEMISTRY CLASS XI
... should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and other properties. Atoms of different elements are different in all respects. Atom is the smallest ...
... should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and other properties. Atoms of different elements are different in all respects. Atom is the smallest ...
1 Unit 2: Atomic Theory Unit Notes Name: Period: ______
... Please watch the following video: Bill Nye Atoms Each subatomic particle has very unique physical properties that will in turn; affect the chemical properties of the atoms they make up. The following reading passage will help you to better understand the structure of the atom. Understanding Atomic S ...
... Please watch the following video: Bill Nye Atoms Each subatomic particle has very unique physical properties that will in turn; affect the chemical properties of the atoms they make up. The following reading passage will help you to better understand the structure of the atom. Understanding Atomic S ...
... form of extra electrons or ”holes” have to be injected into the material. A hole is a position where an electron is missing. When such a hole is filled by an electron jumping in from a neighbouring position, a new hole is created and so on, allowing charge to migrate a long distance. Today conductiv ...
getting started 3.1 hydrocarbons
... 1. A functional group is a structural arrangement of atoms that, because of their electronegativity and bonding type, imparts particular characteristics to the molecule. 2. C=C and C)C bonds are more reactive than C–C bonds because the second and third bonds formed are weaker than the single bonds f ...
... 1. A functional group is a structural arrangement of atoms that, because of their electronegativity and bonding type, imparts particular characteristics to the molecule. 2. C=C and C)C bonds are more reactive than C–C bonds because the second and third bonds formed are weaker than the single bonds f ...
Advanced Chemical Reactions
... that electronegativity is a measure of how tightly atoms hold on to their electrons Atoms with large electronegativity differences form ionic bonds by electron transfers 2Na + Cl2 2NaCl Can be written as 2Na + Cl2 2Na+Cl- ...
... that electronegativity is a measure of how tightly atoms hold on to their electrons Atoms with large electronegativity differences form ionic bonds by electron transfers 2Na + Cl2 2NaCl Can be written as 2Na + Cl2 2Na+Cl- ...
9/10/10 1 Chemistry 121: Atomic and Molecular Chemistry
... • In a neutral atom the number of protons is equal to the number of electrons • The chemical identity of an atom can be determined solely from its atomic number. For example, the atomic number of nitrogen is 7. This means that each neutral nitrogen atom has 7 protons and 7 electrons. In other words ...
... • In a neutral atom the number of protons is equal to the number of electrons • The chemical identity of an atom can be determined solely from its atomic number. For example, the atomic number of nitrogen is 7. This means that each neutral nitrogen atom has 7 protons and 7 electrons. In other words ...
Organic Chemistry - University of California, Riverside
... F attracts electrons more than C in C-F bonds because the electronegativity of F (3.9) is much greater than that of C (2.5). In contrast, C-H bonds are not very polar because the electronegativities of H (2.3) and C (2.5) are about the same. Positive (+) values for the electronegativity differences ...
... F attracts electrons more than C in C-F bonds because the electronegativity of F (3.9) is much greater than that of C (2.5). In contrast, C-H bonds are not very polar because the electronegativities of H (2.3) and C (2.5) are about the same. Positive (+) values for the electronegativity differences ...
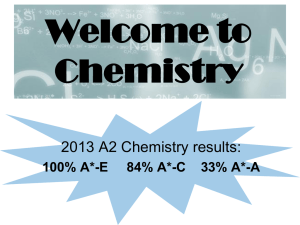
Welcome to Chemistry
... A numerate subject such as CHEMISTRY is useful for… • Accountancy/Business • Architecture • Law ...
... A numerate subject such as CHEMISTRY is useful for… • Accountancy/Business • Architecture • Law ...
Science
... Chemistry should explore the composition of matter through its properties, its atomic structure, and the manner in which it bonds and reacts with other substances. Students should be expected to use suitable mathematics and collect and analyze data. Instruction and assessment should include both app ...
... Chemistry should explore the composition of matter through its properties, its atomic structure, and the manner in which it bonds and reacts with other substances. Students should be expected to use suitable mathematics and collect and analyze data. Instruction and assessment should include both app ...
Student Text, pp. 626-638
... This was a significant discovery. Atoms that will only absorb energy in 4.89-eV packages re-emit the energy in the form of photons of exactly the same energy. In other words, mercury atoms raised to their first excitation level by collisions with electrons de-excite, returning to their ground state ...
... This was a significant discovery. Atoms that will only absorb energy in 4.89-eV packages re-emit the energy in the form of photons of exactly the same energy. In other words, mercury atoms raised to their first excitation level by collisions with electrons de-excite, returning to their ground state ...
Chemistry
... looks a lot like the word “alchemy”. Early alchemists were commonly known as “chemists”, and over time, people started referring to their work, particularly the more legitimate forms of it, as chemistry. While it may seem strange, in many ways it is appropriate that our word for the present-day stud ...
... looks a lot like the word “alchemy”. Early alchemists were commonly known as “chemists”, and over time, people started referring to their work, particularly the more legitimate forms of it, as chemistry. While it may seem strange, in many ways it is appropriate that our word for the present-day stud ...
chemistry
... placed in a test tube rack. The liquids separate into two distinct layers because hexane and water have different (1) formula masses (2) molecular polarities (3) pH values (4) specific heats ...
... placed in a test tube rack. The liquids separate into two distinct layers because hexane and water have different (1) formula masses (2) molecular polarities (3) pH values (4) specific heats ...
Document
... • Was based on electrons having fixed energy levels and therefore quantized amounts of energy. • Accounted for spectral lines. • Worked very well for hydrogen but did not work well for heavier atoms. • Another model is needed that describes the behavior of electrons as waves. Copyright 2012 John Wil ...
... • Was based on electrons having fixed energy levels and therefore quantized amounts of energy. • Accounted for spectral lines. • Worked very well for hydrogen but did not work well for heavier atoms. • Another model is needed that describes the behavior of electrons as waves. Copyright 2012 John Wil ...
1 - Cathedral High School
... energies, electronegativity and melting points for the alkali metals (Li Cs), halogens (F I) and period 3 elements (Na Ar). Cross reference with topics 2, 4 and 5. Data for all these properties are listed in the data booklet. Explanations for the first four trends should be given in terms of t ...
... energies, electronegativity and melting points for the alkali metals (Li Cs), halogens (F I) and period 3 elements (Na Ar). Cross reference with topics 2, 4 and 5. Data for all these properties are listed in the data booklet. Explanations for the first four trends should be given in terms of t ...
Page 1
... 53. How many atoms (molecules), how much volume and how many grams does one mole of chlorine gas have? 1 mol Cl2 = 22.4 Liters 1 mol Cl2 = 6.02 x 1023 molecules 1 mol Cl2 = 70.90 grams 54. State the Law of Conservation of Mass. States that mass is neither created nor destroyed in any process but is ...
... 53. How many atoms (molecules), how much volume and how many grams does one mole of chlorine gas have? 1 mol Cl2 = 22.4 Liters 1 mol Cl2 = 6.02 x 1023 molecules 1 mol Cl2 = 70.90 grams 54. State the Law of Conservation of Mass. States that mass is neither created nor destroyed in any process but is ...
Empirical Formula, Molecular Formula, Percent Composition
... a) You are given the mass of carbon and Liters of hydrogen and oxygen in the molecule. The first step is to convert all of these values to moles of each element because we can only compare them in a mole to mole ratio. 3.60 g carbon x 1 mole carbon/12.01 g = 0.3 moles carbon 16.8 L hydrogen x 1 mole ...
... a) You are given the mass of carbon and Liters of hydrogen and oxygen in the molecule. The first step is to convert all of these values to moles of each element because we can only compare them in a mole to mole ratio. 3.60 g carbon x 1 mole carbon/12.01 g = 0.3 moles carbon 16.8 L hydrogen x 1 mole ...
Chemistry (B) Final Exam Study Guide 3
... compounds that have the same molecular formula, but the atoms are joined in a different order arrangement in which substituted groups are on the same side of a double bond molecules in which atoms are joined in the same order but differ in the arrangements of their atoms in space arrangement in whic ...
... compounds that have the same molecular formula, but the atoms are joined in a different order arrangement in which substituted groups are on the same side of a double bond molecules in which atoms are joined in the same order but differ in the arrangements of their atoms in space arrangement in whic ...
Section 2 Oxidation Numbers
... • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, _________ ________ are assigned to the atoms composing the compound or ion. • Unlike ionic charges, _______ ________ do not have an ______ ________ meaning: rather, ...
... • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, _________ ________ are assigned to the atoms composing the compound or ion. • Unlike ionic charges, _______ ________ do not have an ______ ________ meaning: rather, ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.