GROUP 13 ELEMENTS -THE BORON FAMILY -
... do not shield the nuclear very effectively, so that the orbital electrons are more firmly or tightly held and the metal are less electropositive. This is evidenced by the increase of ionization energy between Al and Ga even though the large atom would be expected to have a lower value ...
... do not shield the nuclear very effectively, so that the orbital electrons are more firmly or tightly held and the metal are less electropositive. This is evidenced by the increase of ionization energy between Al and Ga even though the large atom would be expected to have a lower value ...
mole - hrsbstaff.ednet.ns.ca
... grams of carbon-12. There are 6.02 x 1023 atoms of carbon in 12.0 grams of carbon-12. In one mole, there are 6.02 x 1023 particles; the mole can be treated like a very large dozen. The number 6.02 x 1023 is called Avogadro’s number, named in honor of Amedeo Avogadro, an Italian chemist who first sug ...
... grams of carbon-12. There are 6.02 x 1023 atoms of carbon in 12.0 grams of carbon-12. In one mole, there are 6.02 x 1023 particles; the mole can be treated like a very large dozen. The number 6.02 x 1023 is called Avogadro’s number, named in honor of Amedeo Avogadro, an Italian chemist who first sug ...
1 Course Code– CH1141 Semester – I Credit
... 8. Which is more stable O2 or O22+ ? 9. What is the enthalpy for elementary substances in their standard state? 10. What is the unit of dipole moment? 10x1 = 10 marks ...
... 8. Which is more stable O2 or O22+ ? 9. What is the enthalpy for elementary substances in their standard state? 10. What is the unit of dipole moment? 10x1 = 10 marks ...
When the atom went quantum
... the electron possessed an angular momentum equal to a multiple of Planck’s constant divided by 2 pi. With that constraint, Bohr could explain why light was emitted from hydrogen atoms only in certain very specific colors (or frequencies). An emitted color corresponded to an electron jumping from on ...
... the electron possessed an angular momentum equal to a multiple of Planck’s constant divided by 2 pi. With that constraint, Bohr could explain why light was emitted from hydrogen atoms only in certain very specific colors (or frequencies). An emitted color corresponded to an electron jumping from on ...
Stoichiometry - coercingmolecules
... The mole (n or mol) is the amount of matter that contains as many entities (atoms, molecules, ions, or other particles) as there are atoms in exactly 12 g of the carbon-12 isotope (12C) • The actual number of atoms in 12 g of ...
... The mole (n or mol) is the amount of matter that contains as many entities (atoms, molecules, ions, or other particles) as there are atoms in exactly 12 g of the carbon-12 isotope (12C) • The actual number of atoms in 12 g of ...
Unit 2 Atomic Theories and Structures Packet
... 19)______________________ A scientist whose research lead to the discovery that the nucleus is small, dense, and positively charged. The electrons orbit around the nucleus. The experiment he used is known as the gold foil experiment. 20)______________________ Smallest particle of an element that ret ...
... 19)______________________ A scientist whose research lead to the discovery that the nucleus is small, dense, and positively charged. The electrons orbit around the nucleus. The experiment he used is known as the gold foil experiment. 20)______________________ Smallest particle of an element that ret ...
Redox
... Before metallurgy, humans discovered fire. The technology of fire has been crucial in the development of human cultures, but only relatively recently (18th century) have we come to realize the role of oxygen in burning. Understanding the connection of corrosion (rusting, tarnishing, etc.) and burnin ...
... Before metallurgy, humans discovered fire. The technology of fire has been crucial in the development of human cultures, but only relatively recently (18th century) have we come to realize the role of oxygen in burning. Understanding the connection of corrosion (rusting, tarnishing, etc.) and burnin ...
Stars and Elements
... HOW CAN LOOKING AT THE SAME INFORMATION FROM DIFFERENT PERSPECTIVES PAVE THE WAY FOR PROGRESS? ...
... HOW CAN LOOKING AT THE SAME INFORMATION FROM DIFFERENT PERSPECTIVES PAVE THE WAY FOR PROGRESS? ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... eg. when table salt is mixed with water, it seems to disappear, or become a liquid - the composition can vary from one sample to another. Pure substances have constant composition. Salt water samples from different seas or lakes have different amounts of salt ...
... eg. when table salt is mixed with water, it seems to disappear, or become a liquid - the composition can vary from one sample to another. Pure substances have constant composition. Salt water samples from different seas or lakes have different amounts of salt ...
Study Guide for Final #1
... Topics to Know 1.) Know what extensive and intensive properties are. 2.) Know what the three states of matter are and how matter changes from one state to another. 3.) Know what physical properties are and be able to give examples. 4.) Know what physical changes are and how they are different from c ...
... Topics to Know 1.) Know what extensive and intensive properties are. 2.) Know what the three states of matter are and how matter changes from one state to another. 3.) Know what physical properties are and be able to give examples. 4.) Know what physical changes are and how they are different from c ...
Calculation of the mass of material in a given number of moles of at
... Students should read the introduction and examples at the start of each section then attempt to complete all the questions that are NOT in a shaded box. ...
... Students should read the introduction and examples at the start of each section then attempt to complete all the questions that are NOT in a shaded box. ...
Worksheet Significant Figures
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
38 The Atom and the Quantum
... Each element has a unique arrangement of electron orbits. The radii of orbits for the sodium atom are the same for all sodium atoms, but different from the radii of orbits for other kinds of atoms. Each element has its own distinct orbits. ...
... Each element has a unique arrangement of electron orbits. The radii of orbits for the sodium atom are the same for all sodium atoms, but different from the radii of orbits for other kinds of atoms. Each element has its own distinct orbits. ...
Material particles and light have both wave properties and particle
... Each element has a unique arrangement of electron orbits. The radii of orbits for the sodium atom are the same for all sodium atoms, but different from the radii of orbits for other kinds of atoms. Each element has its own distinct orbits. ...
... Each element has a unique arrangement of electron orbits. The radii of orbits for the sodium atom are the same for all sodium atoms, but different from the radii of orbits for other kinds of atoms. Each element has its own distinct orbits. ...
14 Shape of Spectral Lines
... 14.3 Doppler Broadening of Spectral Lines The atoms that make up the gas of the stellar atmosphere are constantly in motion, and this motion shifts the wavelengths, seen by an observer, at which the atoms can absorb radiation. This motion may be only the thermal motion of the gas, or it may include ...
... 14.3 Doppler Broadening of Spectral Lines The atoms that make up the gas of the stellar atmosphere are constantly in motion, and this motion shifts the wavelengths, seen by an observer, at which the atoms can absorb radiation. This motion may be only the thermal motion of the gas, or it may include ...
Chapter 2
... Which of the following statements regarding Dalton’s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestru ...
... Which of the following statements regarding Dalton’s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestru ...
Ch. 2 PP - Lemon Bay High School
... Which of the following statements regarding Dalton’s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestru ...
... Which of the following statements regarding Dalton’s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestru ...
Balancing Reaction Equations Oxidation State Reduction
... Oxidation: Loss of electrons from an element. Oxidation number increases Reduction: Gain of electrons by an element. Oxidation number decreases ...
... Oxidation: Loss of electrons from an element. Oxidation number increases Reduction: Gain of electrons by an element. Oxidation number decreases ...
chapter 02
... Which of the following statements regarding Dalton’s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestru ...
... Which of the following statements regarding Dalton’s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestru ...
Ch. 2 PP - Lemon Bay High School
... Which of the following statements regarding Dalton’s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestru ...
... Which of the following statements regarding Dalton’s atomic theory are still believed to be true? I. Elements are made of tiny particles called atoms. II. All atoms of a given element are identical. III. A given compound always has the same relative numbers and types of atoms. IV. Atoms are indestru ...
Chemical Formulas and Chemical Compounds
... Chemical Formulas and Chemical Compounds Chapter 7 Review – Answers ...
... Chemical Formulas and Chemical Compounds Chapter 7 Review – Answers ...
Unit3_Stoichiometry_vs2
... Chemical Equations • A chemical change can be represented by a chemical equation where the left hand shows the reactants (what you start with) and the right side shows the products (what you end up with!) • Since matter is neither created nor destroyed, the total number of atoms of each element nee ...
... Chemical Equations • A chemical change can be represented by a chemical equation where the left hand shows the reactants (what you start with) and the right side shows the products (what you end up with!) • Since matter is neither created nor destroyed, the total number of atoms of each element nee ...
4.2 Structure of the Nuclear Atom
... • Three kinds of subatomic particles are electrons, protons, and neutrons. • In the nuclear atom, the protons and neutrons are located in the nucleus. The electrons are distributed around the nucleus and occupy almost all the volume of the atom. ...
... • Three kinds of subatomic particles are electrons, protons, and neutrons. • In the nuclear atom, the protons and neutrons are located in the nucleus. The electrons are distributed around the nucleus and occupy almost all the volume of the atom. ...
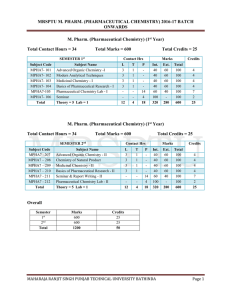
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... John Wiley and Sons, New York. 2. L.G. Chatten, ‘Pharmaceutical Chemistry’, Vol. I & II, Marcel Dekker, New York. 3. W.D. James and H.T. Kenneth, ‘Analytical Chemistry by Open Learning: Thermal Methods. John Wiley and Sons, New York. 4. R.J. Abraham, J. Fisher and P. Bftus, ‘Introduction to NMR Spec ...
... John Wiley and Sons, New York. 2. L.G. Chatten, ‘Pharmaceutical Chemistry’, Vol. I & II, Marcel Dekker, New York. 3. W.D. James and H.T. Kenneth, ‘Analytical Chemistry by Open Learning: Thermal Methods. John Wiley and Sons, New York. 4. R.J. Abraham, J. Fisher and P. Bftus, ‘Introduction to NMR Spec ...
Chapter 3 Molecules, Compounds, and Chemical Equations How
... Covalent Bonds • Covalent bonds—which occur between two or more nonmetals—involve the sharing of electrons between two atoms. • When a nonmetal bonds with another nonmetal, neither atom transfers its electron to the other. Instead the bonding atoms share some of their electrons. ...
... Covalent Bonds • Covalent bonds—which occur between two or more nonmetals—involve the sharing of electrons between two atoms. • When a nonmetal bonds with another nonmetal, neither atom transfers its electron to the other. Instead the bonding atoms share some of their electrons. ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.