atom - SCHOOLinSITES
... •The outermost electron shell of every Noble Gas element (except Helium) has ___ electrons. a) 1; b) 2; c) 4; d) 6; e) 8 •An organic molecule is likely to contain all of these elements except ___. a) C; b) H; c) O; d) Ne; e) N •The chemical bond between water molecules is a ___ bond. a) ionic; b) po ...
... •The outermost electron shell of every Noble Gas element (except Helium) has ___ electrons. a) 1; b) 2; c) 4; d) 6; e) 8 •An organic molecule is likely to contain all of these elements except ___. a) C; b) H; c) O; d) Ne; e) N •The chemical bond between water molecules is a ___ bond. a) ionic; b) po ...
... type of subatomic particle has a different electrical charge. A proton always has an electrical charge of +1. An electron always has an electrical charge of –1. A neutron has no electrical charge associated with it, a charge of 0. Atoms form the building blocks of the simplest substances, the chemic ...
L.O.
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
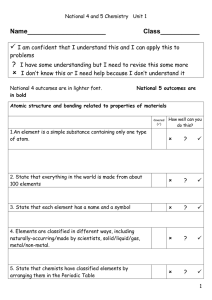
Atomic structure and periodic table notes sheet
... Atomic Structure and Periodic Table Notes 1. What are Atoms? ___________________________________________ ___________________________________________ 2. 400 BC- _________________ was the first scientist to suggest that all matter is composed of tiny, indivisible particles called atoms. (Atomos) 3. El ...
... Atomic Structure and Periodic Table Notes 1. What are Atoms? ___________________________________________ ___________________________________________ 2. 400 BC- _________________ was the first scientist to suggest that all matter is composed of tiny, indivisible particles called atoms. (Atomos) 3. El ...
2-1 Chemistry of life
... • Whole number ratios are like two to one for H2O. Or one two to three for Iron Sulfide Fe2S3 • Dalton’s born to a family of poor Quakers, Same city William Wordsworth comes from, Bright guy, at the age of 12 the put him in charge of the local school • He was color blind and the world called it Dal ...
... • Whole number ratios are like two to one for H2O. Or one two to three for Iron Sulfide Fe2S3 • Dalton’s born to a family of poor Quakers, Same city William Wordsworth comes from, Bright guy, at the age of 12 the put him in charge of the local school • He was color blind and the world called it Dal ...
atom
... 2. All atoms of the same element are exactly alike and have the same mass. Atoms of different elements are different and have different masses. 3. An atom of one element cannot be changed into an atom of a different element. Atoms cannot be created or destroyed in any chemical change, ...
... 2. All atoms of the same element are exactly alike and have the same mass. Atoms of different elements are different and have different masses. 3. An atom of one element cannot be changed into an atom of a different element. Atoms cannot be created or destroyed in any chemical change, ...
Atomic Theory - Hutchk12.org
... To explain the observations of the Gold Foil Experiment the atom must have a very small (less than 0.0001 % of the volume) and very dense (over 99.999% of the mass) particle inside the atom. ...
... To explain the observations of the Gold Foil Experiment the atom must have a very small (less than 0.0001 % of the volume) and very dense (over 99.999% of the mass) particle inside the atom. ...
Chapter 2choutline - Madison County Schools
... experiments. A scientific theory is heavily supported by __________________________ gained from experiments and continual observations. Theories explain laws, which is simply a fact of nature that is _____________________ so often that it becomes accepted as truth. A law ...
... experiments. A scientific theory is heavily supported by __________________________ gained from experiments and continual observations. Theories explain laws, which is simply a fact of nature that is _____________________ so often that it becomes accepted as truth. A law ...
Science Outline - cloudfront.net
... Element: substance made up of one kind of ____________________ o Ex: gold Each Element has an _________________ _________________ o Atomic Number: number of ________________ in 1 atom of an element o Each atom of an element has a specific number of ________________ in its nucleus EX: All Hydro ...
... Element: substance made up of one kind of ____________________ o Ex: gold Each Element has an _________________ _________________ o Atomic Number: number of ________________ in 1 atom of an element o Each atom of an element has a specific number of ________________ in its nucleus EX: All Hydro ...
File
... Dalton’s Atomic Theory 1. All matter is made of invisible and indestructible particles (atoms) 2. Atoms of same element are identical 3. Atoms of different elements differ in physical and chemical properties 4. Atoms of different elements combine in simple whole number ratios to form compounds 5. C ...
... Dalton’s Atomic Theory 1. All matter is made of invisible and indestructible particles (atoms) 2. Atoms of same element are identical 3. Atoms of different elements differ in physical and chemical properties 4. Atoms of different elements combine in simple whole number ratios to form compounds 5. C ...
chapter 2-1 - Doral Academy Preparatory
... How do we identify elements? Atomic number: equal to the number of protons that the atom contains ...
... How do we identify elements? Atomic number: equal to the number of protons that the atom contains ...
Atomic Structure and the Periodic Table
... Chemical Symbols Chemical symbol – an abbreviated way to write the name of an element. -example: Carbon – C Proton – positively charged particle found in the nucleus of an atom. Neutron – neutrally charged particle found in the nucleus of an atom. Atomic number – the number of protons in the nucleu ...
... Chemical Symbols Chemical symbol – an abbreviated way to write the name of an element. -example: Carbon – C Proton – positively charged particle found in the nucleus of an atom. Neutron – neutrally charged particle found in the nucleus of an atom. Atomic number – the number of protons in the nucleu ...
File
... This piece would be indivisible. He named the smallest piece of matter “atomos,” meaning “not to be cut.” ...
... This piece would be indivisible. He named the smallest piece of matter “atomos,” meaning “not to be cut.” ...
atomic number
... 2.1 THE ATOMIC THEORY OF MATTER Material world made up of tiny indivisible particles they called atomos, meaning “indivisible or uncuttable.” Dalton’s Atomic Theory Dalton’s atomic theory was based on the four postulates 1. Each element is composed of extremely small particles called atoms. ...
... 2.1 THE ATOMIC THEORY OF MATTER Material world made up of tiny indivisible particles they called atomos, meaning “indivisible or uncuttable.” Dalton’s Atomic Theory Dalton’s atomic theory was based on the four postulates 1. Each element is composed of extremely small particles called atoms. ...
Vocabulary
... • Greek prefixes indicate the number of atoms in each molecule • If the first element noted is one, “mono” is left out, e.g. carbon dioxide = CO2 • Mono – one Hexa – six • Di – two Hepta – seven • Tri – three Octo – eight • Tetra – four Nona – nine • Penta – five Deca – ten ...
... • Greek prefixes indicate the number of atoms in each molecule • If the first element noted is one, “mono” is left out, e.g. carbon dioxide = CO2 • Mono – one Hexa – six • Di – two Hepta – seven • Tri – three Octo – eight • Tetra – four Nona – nine • Penta – five Deca – ten ...
The Atom - Effingham County Schools
... Modern Atomic Theory Not all aspects of Dalton’s atomic theory have proven to be correct. We now know that: • Atoms are divisible into even smaller particles • A given element can have atoms with different masses Some important concepts remain unchanged • All matter is composed of atoms • Atom ...
... Modern Atomic Theory Not all aspects of Dalton’s atomic theory have proven to be correct. We now know that: • Atoms are divisible into even smaller particles • A given element can have atoms with different masses Some important concepts remain unchanged • All matter is composed of atoms • Atom ...
Webquest: Atomic Theories and Models Answer these questions on
... 6. In 1909 this scientist demonstrated that the atom is mostly empty space with a small positively charged nucleus containing most of the mass and low mass negatively charged particles orbiting this nucleus. He was also credited with naming alpha and beta radiation/particles. 7. What date did Neils ...
... 6. In 1909 this scientist demonstrated that the atom is mostly empty space with a small positively charged nucleus containing most of the mass and low mass negatively charged particles orbiting this nucleus. He was also credited with naming alpha and beta radiation/particles. 7. What date did Neils ...
Atomic mass
... The Mole, Avogadro’s Number, and Molar Mass mole – the amount of a substance that contains the same number of particles as the number of atoms in exactly 12 g of carbon-12. Abbreviation is mol. Avogadro’s number – 6.022 x 1023 – the number of particles in exactly one mole of a pure substance. Molar ...
... The Mole, Avogadro’s Number, and Molar Mass mole – the amount of a substance that contains the same number of particles as the number of atoms in exactly 12 g of carbon-12. Abbreviation is mol. Avogadro’s number – 6.022 x 1023 – the number of particles in exactly one mole of a pure substance. Molar ...
- Trinity Regional School
... the valence shell and is often unstable, meaning It does not contain the octet number of electrons In order for the atom to become stable or Fill the shell to the octet rule, this shell will be The one to bond. ...
... the valence shell and is often unstable, meaning It does not contain the octet number of electrons In order for the atom to become stable or Fill the shell to the octet rule, this shell will be The one to bond. ...
Basic structure of atoms
... • Electrons move very rapidly in complicated paths called orbitals. • Because of this motion, they appear to form a cloud. – Negative charge -1 – Mass: 9.1 x10-28 grams – Symbols include e-, -1e0 ...
... • Electrons move very rapidly in complicated paths called orbitals. • Because of this motion, they appear to form a cloud. – Negative charge -1 – Mass: 9.1 x10-28 grams – Symbols include e-, -1e0 ...
ATOMS
... and other properties; atoms of different elements differ in size, mass, and other properties Atoms cannot be subdivided, created, or destroyed Atoms of different elements combine in simple wholenumber ratios to form chemical compounds In chemical reactions, atoms are combined, separated, or rearrang ...
... and other properties; atoms of different elements differ in size, mass, and other properties Atoms cannot be subdivided, created, or destroyed Atoms of different elements combine in simple wholenumber ratios to form chemical compounds In chemical reactions, atoms are combined, separated, or rearrang ...
atom
... thought that you would eventually end up with a particle that could not be cut. He called this particle an atom. The word atom comes from the Greek word atomos, meaning “not able to be divided”. Democritus said that all atoms are small, hard particles. He thought that atoms were made of a single mat ...
... thought that you would eventually end up with a particle that could not be cut. He called this particle an atom. The word atom comes from the Greek word atomos, meaning “not able to be divided”. Democritus said that all atoms are small, hard particles. He thought that atoms were made of a single mat ...
Document
... then the theory of atomic structure. They used massive alpha particles. Alpha Particles- helium atoms that lost two electrons. double positive charge due to remaining protons. The belief was that the Alpha particles would pass easily though the gold with only slight deflection. This was said to happ ...
... then the theory of atomic structure. They used massive alpha particles. Alpha Particles- helium atoms that lost two electrons. double positive charge due to remaining protons. The belief was that the Alpha particles would pass easily though the gold with only slight deflection. This was said to happ ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.