CHEMISTRY IM 06 SYLLABUS
... Section B will consist of five compulsory structured questions; Section C will require candidates to choose two out of four long questions. Each section carries equal marks. The minimum mathematical requirements of the syllabus are the same as those for the SEC examination in Chemistry. Questions wi ...
... Section B will consist of five compulsory structured questions; Section C will require candidates to choose two out of four long questions. Each section carries equal marks. The minimum mathematical requirements of the syllabus are the same as those for the SEC examination in Chemistry. Questions wi ...
CHEMISTRY IM 06 SYLLABUS
... Section B will consist of five compulsory structured questions; Section C will require candidates to choose two out of four long questions. Each section carries equal marks. The minimum mathematical requirements of the syllabus are the same as those for the SEC examination in Chemistry. Questions wi ...
... Section B will consist of five compulsory structured questions; Section C will require candidates to choose two out of four long questions. Each section carries equal marks. The minimum mathematical requirements of the syllabus are the same as those for the SEC examination in Chemistry. Questions wi ...
Bonds - Cloudfront.net
... Section 2 Types of Bonds Covalent Bonds The attraction that forms between atoms when they share electrons is known as a covalent bond A neutral particle that forms as a result of electron sharing is called a molecule Atoms can form double and triple bonds depending on whether they share two o ...
... Section 2 Types of Bonds Covalent Bonds The attraction that forms between atoms when they share electrons is known as a covalent bond A neutral particle that forms as a result of electron sharing is called a molecule Atoms can form double and triple bonds depending on whether they share two o ...
Itty-Bitty Atoms
... science? Would you like to be a scientist? If so, what would you like to study? What do you think scientists of the future will study? 4. Answer the following questions: a. Who is Dmitry Mendeleyev and what did he do? b. What is chemistry? c. How big are atoms? 5. When a teacher calls out a symbol f ...
... science? Would you like to be a scientist? If so, what would you like to study? What do you think scientists of the future will study? 4. Answer the following questions: a. Who is Dmitry Mendeleyev and what did he do? b. What is chemistry? c. How big are atoms? 5. When a teacher calls out a symbol f ...
I. Why Atoms Combine - Manchester High School
... Write the names of both elements. Change the final ending to -ide. ...
... Write the names of both elements. Change the final ending to -ide. ...
PS Unit 3 Part 1 Notes
... • Could not answer question “What holds atoms together?” Aristotle • Most influential Greek philosopher (384 – 322 BC) • Rejected atomic theory of Democritus because it conflicted with his ideas on nature • Did not believe that “nothingness” of empty space could exist ...
... • Could not answer question “What holds atoms together?” Aristotle • Most influential Greek philosopher (384 – 322 BC) • Rejected atomic theory of Democritus because it conflicted with his ideas on nature • Did not believe that “nothingness” of empty space could exist ...
File - A. Hall Teaching Site
... Concepts: The atomic story starts with Democritus. He named the atom with the word atomos when he first proposed the idea of matter being made up of tiny particles. He determined that atoms cannot be divided, created or destroyed. He also determined that there is empty space between atoms. Democritu ...
... Concepts: The atomic story starts with Democritus. He named the atom with the word atomos when he first proposed the idea of matter being made up of tiny particles. He determined that atoms cannot be divided, created or destroyed. He also determined that there is empty space between atoms. Democritu ...
Atomic Structure - s3.amazonaws.com
... https://www.youtube.com/watch?v=fPnwB ITSmgU Predicting the Elements ...
... https://www.youtube.com/watch?v=fPnwB ITSmgU Predicting the Elements ...
CH. 15 Notes
... Caution: This is a lethal substance (kills thousands of people each year), can be used for rocket fuel, causes billions of dollars in damage, found everywhere ...
... Caution: This is a lethal substance (kills thousands of people each year), can be used for rocket fuel, causes billions of dollars in damage, found everywhere ...
Section 3
... SECTION 3.1 Democritus – was a fourth century B.C. Greek philosopher who proposed that the universe was made up of tiny particles called atoms. The word atom was derived from the Greek word Atomos, “meaning indivisible or indestructible”. John Dalton’s Atomic Theory •Every element is made of tiny, u ...
... SECTION 3.1 Democritus – was a fourth century B.C. Greek philosopher who proposed that the universe was made up of tiny particles called atoms. The word atom was derived from the Greek word Atomos, “meaning indivisible or indestructible”. John Dalton’s Atomic Theory •Every element is made of tiny, u ...
Test Review: Unit 1 - Ms. Hill`s Pre
... a. Law of conservation of Mass: Mass is neither created nor destroyed during normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain ...
... a. Law of conservation of Mass: Mass is neither created nor destroyed during normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain ...
Atomic Structure-1
... Rutherford’s Atom Rutherford’s Planetary Model (1911) 〉 Intended to study Thomson’s model. 〉 Conducted an experiment with gold foil and alpha particles. ...
... Rutherford’s Atom Rutherford’s Planetary Model (1911) 〉 Intended to study Thomson’s model. 〉 Conducted an experiment with gold foil and alpha particles. ...
10.1 – District 1: Atomic Theory Key Points Notes Atomic Structure
... Where are these found in the atom? ...
... Where are these found in the atom? ...
Unit 3 Chap. 3 Atoms: The Building Blocks of Matter
... Greeks four fundamental substances: fire, earth, water, and air; Demokritos - “atomos” alchemy - discovered some elements and mineral acids Robert Boyle - relationship btw. P & V George Stahl - “phlogiston” from combustion Joseph Priestley - discovered oxygen Antoine Lavoisier - discovered combustio ...
... Greeks four fundamental substances: fire, earth, water, and air; Demokritos - “atomos” alchemy - discovered some elements and mineral acids Robert Boyle - relationship btw. P & V George Stahl - “phlogiston” from combustion Joseph Priestley - discovered oxygen Antoine Lavoisier - discovered combustio ...
Physical Science Chapter 4 Study Guide mod 5
... 10. Rutherford’s model determined that an atom’s positive charge is concentrated in the atom’s center. 11. True or false: Neutrons have a positive charge? False 12. Thomson discovered that an atom contains negatively charged particles (electrons). 13. What did Thomson use to make his discovery abou ...
... 10. Rutherford’s model determined that an atom’s positive charge is concentrated in the atom’s center. 11. True or false: Neutrons have a positive charge? False 12. Thomson discovered that an atom contains negatively charged particles (electrons). 13. What did Thomson use to make his discovery abou ...
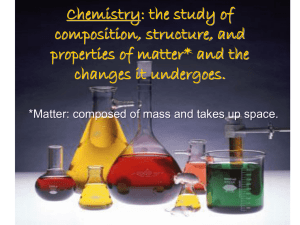
Chemistry: the study of composition, structure, and properties of
... Chemistry: the study of composition, structure, and properties of matter* and the changes it undergoes. *Matter: composed of mass and takes up space. ...
... Chemistry: the study of composition, structure, and properties of matter* and the changes it undergoes. *Matter: composed of mass and takes up space. ...
Chapter 3: Atoms and Moles
... What are the building blocks of all matter? 400 BC : Democritus came up with the idea that there’s a limit to how much you can divide something; There is a unit that is indivisible, the atom (atomic theory) ...
... What are the building blocks of all matter? 400 BC : Democritus came up with the idea that there’s a limit to how much you can divide something; There is a unit that is indivisible, the atom (atomic theory) ...
32__atom_quantum____..
... D) one half as large. E) one quarter as large. 12) A new theory conforms to the correspondence principle when it A) ties two or more theories together. B) corresponds to all theories in nature. C) updates the essence of the old theory. D) accounts for verified results of the old theory. E) none of t ...
... D) one half as large. E) one quarter as large. 12) A new theory conforms to the correspondence principle when it A) ties two or more theories together. B) corresponds to all theories in nature. C) updates the essence of the old theory. D) accounts for verified results of the old theory. E) none of t ...
3 UE Act 2ab PPT History of Atomic Theory.cd
... He asked: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? ...
... He asked: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? ...
Chapter 2 Atoms, Ions, and Molecules
... 3. atoms of different elements have different masses, physical properties, and chemical properties 4. atoms of different elements combine in simple whole numbers to form compounds 5. atoms of an element cannot be converted into atoms of other elements; chemical reactions involve reorganization of th ...
... 3. atoms of different elements have different masses, physical properties, and chemical properties 4. atoms of different elements combine in simple whole numbers to form compounds 5. atoms of an element cannot be converted into atoms of other elements; chemical reactions involve reorganization of th ...
Chem12-Unit 1-1-Development of Atomic Theory
... Solid-Particle Model (1805) Democritus coined the word “Atom”. Dalton’s Atomic Theory: 1. Atoms are the basic particles of matter. 2. They are indivisible and cannot be created or destroyed. 3. Atoms of a given element are identical, having the same mass and the same chemical properties. 4. Atoms of ...
... Solid-Particle Model (1805) Democritus coined the word “Atom”. Dalton’s Atomic Theory: 1. Atoms are the basic particles of matter. 2. They are indivisible and cannot be created or destroyed. 3. Atoms of a given element are identical, having the same mass and the same chemical properties. 4. Atoms of ...
Chapter 5: Atomic Structure & The Periodic Table
... Chapter 5: Atomic Structure & The Periodic Table Democritus– 4th century B.C., teacher in Greece, first suggested the existence of atoms, lacked experimental support because scientific testing was unknown at the time. 2000 years after Democritus, the real nature of atoms and observable changes at th ...
... Chapter 5: Atomic Structure & The Periodic Table Democritus– 4th century B.C., teacher in Greece, first suggested the existence of atoms, lacked experimental support because scientific testing was unknown at the time. 2000 years after Democritus, the real nature of atoms and observable changes at th ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.