PS_Module 4 - Leon County Schools
... • The law of conservation of mass is also true for physical changes. An ice cube has the same mass as the water produced when it melts. • In other cases, conservation of mass is less obvious. If we think about it, we can usually explain cases in which the law of conservation of mass seems to have be ...
... • The law of conservation of mass is also true for physical changes. An ice cube has the same mass as the water produced when it melts. • In other cases, conservation of mass is less obvious. If we think about it, we can usually explain cases in which the law of conservation of mass seems to have be ...
Models of the Atom - Central Magnet School
... Rutherford called this region the nucleus. Particles that approach the nucleus closely are greatly ...
... Rutherford called this region the nucleus. Particles that approach the nucleus closely are greatly ...
Atomic - Ms. Dawkins
... What is the structure of an atom? • The protons and neutrons are grouped together in the center of the atom. • The center of the atom is called the nucleus. • Electrons move around outside the nucleus in what we call an electron cloud. • The nucleus has an overall positive charge (because it contai ...
... What is the structure of an atom? • The protons and neutrons are grouped together in the center of the atom. • The center of the atom is called the nucleus. • Electrons move around outside the nucleus in what we call an electron cloud. • The nucleus has an overall positive charge (because it contai ...
Chemistry Unit Objectives 2.1a Recognize that the Periodic Table is
... 2.2b Identify and describe that when two or more atoms chemically combine, they either share electrons (covalent bond, which can be polar or non-polar) or transfer electrons (ionic bond). -Explain how and why atoms form ions and combine in ionic bonds through the transfer of electrons. -Explain how ...
... 2.2b Identify and describe that when two or more atoms chemically combine, they either share electrons (covalent bond, which can be polar or non-polar) or transfer electrons (ionic bond). -Explain how and why atoms form ions and combine in ionic bonds through the transfer of electrons. -Explain how ...
Development of Atomic Theory - Phillips Scientific Methods
... Dalton definite proportions electron Goldstein ...
... Dalton definite proportions electron Goldstein ...
Atoms - Chemistry Land
... cold beaker above it, you can see water condensing on it. So the dry paper actually has water in it. Finally as it burns out you see the ashes, which is like dirt or earth. ...
... cold beaker above it, you can see water condensing on it. So the dry paper actually has water in it. Finally as it burns out you see the ashes, which is like dirt or earth. ...
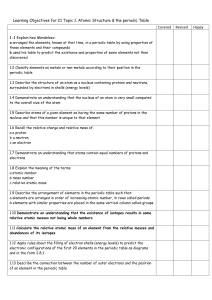
C2- Topic 1: Atomic structure and the periodic table. Assessable
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
atomic
... electron (e-) -negatively charged subatomic particle -found surrounding the nucleus in energy levels or electron clouds -discovered by JJ Thomson in 1897 -mass= 9.11 X 10-28g ...
... electron (e-) -negatively charged subatomic particle -found surrounding the nucleus in energy levels or electron clouds -discovered by JJ Thomson in 1897 -mass= 9.11 X 10-28g ...
The Nuclear Atom
... Aristotle (460 B.C. – 370 B.C.) • emphasized that nature consisted of four elements: air, earth, fire, and water • did not believe in discontinuous or separate atoms, but felt that matter was continuous ...
... Aristotle (460 B.C. – 370 B.C.) • emphasized that nature consisted of four elements: air, earth, fire, and water • did not believe in discontinuous or separate atoms, but felt that matter was continuous ...
C2 Topic 1 Can Do Sheet
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
Chemical Composition
... • If I want to know how many O2 molecules I will need or how many CO2 molecules I can make, I will need to know how many C atoms are in the sample of carbon I am starting with • Dalton used the percentages of elements in compounds and the chemical formulas to deduce the relative masses of atoms • Un ...
... • If I want to know how many O2 molecules I will need or how many CO2 molecules I can make, I will need to know how many C atoms are in the sample of carbon I am starting with • Dalton used the percentages of elements in compounds and the chemical formulas to deduce the relative masses of atoms • Un ...
Periodic Trends
... – Malleable: hammered into thin sheets – Good conductors of heat/electricity – Luster: shine – Solid at room temperature (except for Hg) ...
... – Malleable: hammered into thin sheets – Good conductors of heat/electricity – Luster: shine – Solid at room temperature (except for Hg) ...
PKUESJX Grade 10 Chemistry Pre
... Student knowledge will be assessed internally through homework and end of topic tests. Skills acquisition will be assessed through two experimental reports and a homework assignment on “Using Elements in Industry”, which will be introduced during Unit Two. ...
... Student knowledge will be assessed internally through homework and end of topic tests. Skills acquisition will be assessed through two experimental reports and a homework assignment on “Using Elements in Industry”, which will be introduced during Unit Two. ...
Polar Covalent bonds
... One electron from each. atom is shared but not equally due to unequal attraction (electronegativity) for shared electrons The bond is referred to as polar because 2 poles are formed (+ and -) Electronegativity values allows us to determine which atom has a greater pull The atom with the greater elec ...
... One electron from each. atom is shared but not equally due to unequal attraction (electronegativity) for shared electrons The bond is referred to as polar because 2 poles are formed (+ and -) Electronegativity values allows us to determine which atom has a greater pull The atom with the greater elec ...
Atomic Structure
... A neutral phosphorus atom has 15 electrons that exist in three shells. The arrangement of electrons in these shells is 2, 8, 5. Draw an X for each electron on the shells below to represent each of the 15 electrons in phosphorus, indicating how many electrons will be found in the first, second, and t ...
... A neutral phosphorus atom has 15 electrons that exist in three shells. The arrangement of electrons in these shells is 2, 8, 5. Draw an X for each electron on the shells below to represent each of the 15 electrons in phosphorus, indicating how many electrons will be found in the first, second, and t ...
4.1 Studying Atoms PDF
... Ø Proposed theory (model) stating that Ø All matter is made up of individual particles ...
... Ø Proposed theory (model) stating that Ø All matter is made up of individual particles ...
Looking for Patterns in Chemical Reactivity
... to the energy changes that take place when their atoms lose, gain, or share electrons to obtain a filled valence shell. Metals are elements that tend to lose their valence electrons relatively easily and this accounts for many of their physical and chemical properties. One important property of ...
... to the energy changes that take place when their atoms lose, gain, or share electrons to obtain a filled valence shell. Metals are elements that tend to lose their valence electrons relatively easily and this accounts for many of their physical and chemical properties. One important property of ...
Section 6.1 Atoms and Moles C. The Mole
... How is this relevant to counting the number of atoms? • Atoms of the same element can have different masses (isotopes) ...
... How is this relevant to counting the number of atoms? • Atoms of the same element can have different masses (isotopes) ...
Atoms and elements Metals and non-metals
... 2. Work out which group the element is in and draw that number of electrons in the outer circle – with eight for Group 0 elements – except helium. 3. Fill the other circles with as many electrons as needed. Remember – two in the first circle, and eight in the second and third circles. 4. Finally, ch ...
... 2. Work out which group the element is in and draw that number of electrons in the outer circle – with eight for Group 0 elements – except helium. 3. Fill the other circles with as many electrons as needed. Remember – two in the first circle, and eight in the second and third circles. 4. Finally, ch ...
View PDF
... 6. In an atomic model that includes a nucleus, positive charge is a. concentrated in the center of an atom. b. spread evenly throughout an atom. c. concentrated at multiple sites in an atom. d. located in the space outside the nucleus. 7. Which subatomic particle has a negative charge? a. electron b ...
... 6. In an atomic model that includes a nucleus, positive charge is a. concentrated in the center of an atom. b. spread evenly throughout an atom. c. concentrated at multiple sites in an atom. d. located in the space outside the nucleus. 7. Which subatomic particle has a negative charge? a. electron b ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.