ppt - Wits Structural Chemistry
... Formula weight and molecular weight are virtually identical and are used interchangeably. When dealing with an ionic compound (3D lattice) we do not use molecular formulas to name them, and so we could not calculate a molecular weight. In this case we use the formula weight. ...
... Formula weight and molecular weight are virtually identical and are used interchangeably. When dealing with an ionic compound (3D lattice) we do not use molecular formulas to name them, and so we could not calculate a molecular weight. In this case we use the formula weight. ...
Oxidation-Reduction Reactions
... • How are cations and anions formed? • What determines whether a covalent bond between two atoms is represented by equal sharing or unequal sharing of the bonding electrons? An iron shovel left out in the rain will eventually begin to rust. What is happening to the shovel, and can anything be done t ...
... • How are cations and anions formed? • What determines whether a covalent bond between two atoms is represented by equal sharing or unequal sharing of the bonding electrons? An iron shovel left out in the rain will eventually begin to rust. What is happening to the shovel, and can anything be done t ...
Chemistry - WorkNotes
... The following released test questions are taken from the Chemistry Standards Test. This test is one of the California Standards Tests administered as part of the Standardized Testing and Reporting (STAR) Program under policies set by the State Board of Education. All questions on the California Stan ...
... The following released test questions are taken from the Chemistry Standards Test. This test is one of the California Standards Tests administered as part of the Standardized Testing and Reporting (STAR) Program under policies set by the State Board of Education. All questions on the California Stan ...
Stoichiometry and the Mole
... • 3 Al(s) + 3 NH4ClO4 Al2O3 + AlCl3 + 3 NO + 6H2O • How much Al must be used to react with 652 g of NH4ClO4 ? • How much water is produced? • How much AlCl3? ...
... • 3 Al(s) + 3 NH4ClO4 Al2O3 + AlCl3 + 3 NO + 6H2O • How much Al must be used to react with 652 g of NH4ClO4 ? • How much water is produced? • How much AlCl3? ...
The Periodic Law and Ionic Charge
... The sulfur atom is two shy of a full 3rd energy level, so in addition to the two additional electrons needed to fill the 3rd energy level, all new electrons get added to the 4s(2), the 3d(10), and then 4 more to get to the same valence configuration as O and S. (18 total) 3. Lead generally forms ion ...
... The sulfur atom is two shy of a full 3rd energy level, so in addition to the two additional electrons needed to fill the 3rd energy level, all new electrons get added to the 4s(2), the 3d(10), and then 4 more to get to the same valence configuration as O and S. (18 total) 3. Lead generally forms ion ...
Unit 5: Chemical Equations and Reactions
... 2. For hydrocarbon combustion, balance in the order of C, H, and then O. The product, H2O, is always in gaseous form unless otherwise stated. (It’s usually quite hot in combustion.) 3. For other type of reactions, balance the equation for each type of cations and anions. Do NOT break up the polyatom ...
... 2. For hydrocarbon combustion, balance in the order of C, H, and then O. The product, H2O, is always in gaseous form unless otherwise stated. (It’s usually quite hot in combustion.) 3. For other type of reactions, balance the equation for each type of cations and anions. Do NOT break up the polyatom ...
Chapter 11 - Cloudfront.net
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
Principles of Chemistry 1 and 2 Notes
... a. Draw Lewis structure of the compound. a. Count # of bonding pairs (central atom / terminal atoms). a. Count # of lone pairs (nonbonding); (around the central atoms ONLY) a. Look at the tables 10.1 and 10.2 (pages 369 and 375, respectively) in the textbook and figure out the electron bonding pair ...
... a. Draw Lewis structure of the compound. a. Count # of bonding pairs (central atom / terminal atoms). a. Count # of lone pairs (nonbonding); (around the central atoms ONLY) a. Look at the tables 10.1 and 10.2 (pages 369 and 375, respectively) in the textbook and figure out the electron bonding pair ...
Mendelevium
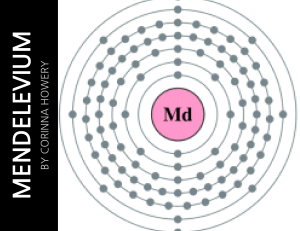
... l Mendelevium (Md) is number 101 on the periodic table so its atomic number is 101. There are 101 protons/electrons in the nucleus and 157 neutrons. It also has 2 valence electrons. Mendelevium has 7 shells. On the periodic table, mendelevium is in the group actinide and it is radioactive. Mendelevi ...
... l Mendelevium (Md) is number 101 on the periodic table so its atomic number is 101. There are 101 protons/electrons in the nucleus and 157 neutrons. It also has 2 valence electrons. Mendelevium has 7 shells. On the periodic table, mendelevium is in the group actinide and it is radioactive. Mendelevi ...
Chemistry Content Review Notes
... The figure above shows a compound containing hydrogen (H) and an unknown element Z. To which group on the periodic table does element Z belong? 5. Bonding between two elements of equal electronegativity would bea. 100% covalent b. Primarily ionic c. 50% ionic d. Metallic in character 6. The small at ...
... The figure above shows a compound containing hydrogen (H) and an unknown element Z. To which group on the periodic table does element Z belong? 5. Bonding between two elements of equal electronegativity would bea. 100% covalent b. Primarily ionic c. 50% ionic d. Metallic in character 6. The small at ...
chapter 8 - Denton ISD
... gas for cooking in your house. Some reactions involve only heat or only light. But heat or light by itself is not necessarily a sign of chemical change, because many physical changes also involve either heat or light. However, heat and light are very strong indicators of a chemical change and should ...
... gas for cooking in your house. Some reactions involve only heat or only light. But heat or light by itself is not necessarily a sign of chemical change, because many physical changes also involve either heat or light. However, heat and light are very strong indicators of a chemical change and should ...
chapter 21 chemistry of the main-group elements i
... the high polarizing power of Be2+, it is difficult to remove the coordinated water molecules by heating the solid and the acidity of the coordinated H2O molecules is enhanced. When BeCl2 4H 2 O is dissolved in water, [Be(H2O)4]2+ ions react with water, and [Be(H2O)3(OH)]+ and H3O+ ions are produce ...
... the high polarizing power of Be2+, it is difficult to remove the coordinated water molecules by heating the solid and the acidity of the coordinated H2O molecules is enhanced. When BeCl2 4H 2 O is dissolved in water, [Be(H2O)4]2+ ions react with water, and [Be(H2O)3(OH)]+ and H3O+ ions are produce ...
The Periodic Table
... levels of the hydrogen atom and postulates only certain allowed circular orbits for the electron. • As the electron becomes more tightly bound, its energy becomes more negative relative to the zero-energy reference state (free electron). As the electron is brought closer to the nucleus, energy is re ...
... levels of the hydrogen atom and postulates only certain allowed circular orbits for the electron. • As the electron becomes more tightly bound, its energy becomes more negative relative to the zero-energy reference state (free electron). As the electron is brought closer to the nucleus, energy is re ...
Compounds
... Binary compounds composed of two nonmetals are usually molecular and are named using a prefix system. Common names- water (H2O) and ammonia(NH3) Write the full name of the first element in the formula. Write the name of the second element in the formula with an ide suffix. Use a prefix in front of e ...
... Binary compounds composed of two nonmetals are usually molecular and are named using a prefix system. Common names- water (H2O) and ammonia(NH3) Write the full name of the first element in the formula. Write the name of the second element in the formula with an ide suffix. Use a prefix in front of e ...
chm 205 - National Open University of Nigeria
... alternate atom in it is Si in place of carbon. In diamond, the strong covalent bonds formed within the giant macromolecule result in a structure which is without any mobile electrons and thus it behaves as an insulator. The rigid, three dimensional linkages make diamond one of the hardest substances ...
... alternate atom in it is Si in place of carbon. In diamond, the strong covalent bonds formed within the giant macromolecule result in a structure which is without any mobile electrons and thus it behaves as an insulator. The rigid, three dimensional linkages make diamond one of the hardest substances ...
FREE Sample Here - We can offer most test bank and
... 25. Which of the following enable us characterize a compound by a specific chemical formula? a. law of conservation of energy c. law of constant composition b. law of conservation of mass d. all of the above ANS: C PTS: 1 TOP: 2.3 - WHAT ARE THE POSTULATES OF DALTON’S ATOMIC THEORY? 26. Which of the ...
... 25. Which of the following enable us characterize a compound by a specific chemical formula? a. law of conservation of energy c. law of constant composition b. law of conservation of mass d. all of the above ANS: C PTS: 1 TOP: 2.3 - WHAT ARE THE POSTULATES OF DALTON’S ATOMIC THEORY? 26. Which of the ...
FREE Sample Here
... In the early 1900s, Ernest Rutherford performed an experiment with thin foils of gold and alpha particles to probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have ...
... In the early 1900s, Ernest Rutherford performed an experiment with thin foils of gold and alpha particles to probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have ...
theodore l. brown h. eugene lemay, jr. bruce e. bursten catherine j
... system, or transmission in any form or by any means, electronic, mechanical, photocopying, recording, or likewise. To obtain permission(s) to use material from this work, please submit a written request to Pearson Education, Inc., Permissions Department, 1900 E. Lake Ave., Glenview, IL 60025. Many o ...
... system, or transmission in any form or by any means, electronic, mechanical, photocopying, recording, or likewise. To obtain permission(s) to use material from this work, please submit a written request to Pearson Education, Inc., Permissions Department, 1900 E. Lake Ave., Glenview, IL 60025. Many o ...
Overhead Notes Stoichiometry
... volumes of gas, at the same temperature and pressure contain the same number of particles. Moles are numbers of particles You can treat reactions as if they happen liters at a time, as long as you keep the temperature and pressure the same. 1 mole = 22.4 L @ STP ...
... volumes of gas, at the same temperature and pressure contain the same number of particles. Moles are numbers of particles You can treat reactions as if they happen liters at a time, as long as you keep the temperature and pressure the same. 1 mole = 22.4 L @ STP ...
Rules for Computing Symmetry, Density and Stoichiometry in a
... Lemma II: Some decagonal quasi-unit-cell models are not equivalent to rhombus Penrose-tile model with the same edge length. Proof: This Lemma asserts that the converse of Lemma I is not true. Consider a configuration obtained by placing one atom at the center of the fundamental decagon as shown in F ...
... Lemma II: Some decagonal quasi-unit-cell models are not equivalent to rhombus Penrose-tile model with the same edge length. Proof: This Lemma asserts that the converse of Lemma I is not true. Consider a configuration obtained by placing one atom at the center of the fundamental decagon as shown in F ...
topic: chemical formula, chemical equations and stoichiometry
... Chemical formula is a representation of a chemical substances using letters for atoms and subscript number to show the numbers of each type of atoms that are present in the substances. Example; Chemical for water: H2O, (H represent for hydrogen atom, O represent for oxygen atom and subscript 2 repre ...
... Chemical formula is a representation of a chemical substances using letters for atoms and subscript number to show the numbers of each type of atoms that are present in the substances. Example; Chemical for water: H2O, (H represent for hydrogen atom, O represent for oxygen atom and subscript 2 repre ...
Practice Test Material - Directorate of Education
... On adding conc. H2SO4 to NaCl(s), HCl gas is produced but Br2(g) and not HBr(g) is obtained when conc. H2SO4 is added to NaBr(s). ...
... On adding conc. H2SO4 to NaCl(s), HCl gas is produced but Br2(g) and not HBr(g) is obtained when conc. H2SO4 is added to NaBr(s). ...
Inorganometallic Chemistry
... compounds are those in which the carbon atoms are bonded to any other element with exception of H, C, N, O, F, Cl, Br, I and At. Some difficulties arise in defining the metal of the main group (p-block) elements. Usually organometallic compounds are comprised not only of compounds of typical metals, ...
... compounds are those in which the carbon atoms are bonded to any other element with exception of H, C, N, O, F, Cl, Br, I and At. Some difficulties arise in defining the metal of the main group (p-block) elements. Usually organometallic compounds are comprised not only of compounds of typical metals, ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... the component that keeps its state is called the solvent if both components start in the same state, the major component is the solvent Tro, Chemistry: A Molecular Approach ...
... the component that keeps its state is called the solvent if both components start in the same state, the major component is the solvent Tro, Chemistry: A Molecular Approach ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.