- sartep.com
... 30. __________ Which symbol represents the atom with the largest number of neutrons? (B) 238U (C) 208Pb (D) 226Ra (E) 237Np (A) 234Th 31. __________ How many orbitals are there in the third energy level? (A) 3 (B) 4 (C) 9 (D) 18 (E) 28 32. __________ How many orbitals in a ground state fluorine atom ...
... 30. __________ Which symbol represents the atom with the largest number of neutrons? (B) 238U (C) 208Pb (D) 226Ra (E) 237Np (A) 234Th 31. __________ How many orbitals are there in the third energy level? (A) 3 (B) 4 (C) 9 (D) 18 (E) 28 32. __________ How many orbitals in a ground state fluorine atom ...
Chapter 3 2013
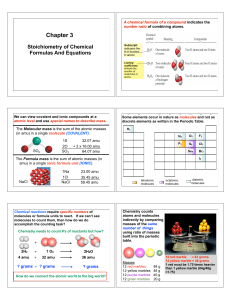
... What’s In A Chemical Formula? Urea, (NH2)2CO, is a nitrogen containing compound used as a fertilizer around the globe? Calculate the following for 25.6 g of urea: a) the molar mass of urea? b) the number of moles of urea in 25.6 g urea? b) # of molecules of urea in 25.6 g of urea? c) # hydrogen ato ...
... What’s In A Chemical Formula? Urea, (NH2)2CO, is a nitrogen containing compound used as a fertilizer around the globe? Calculate the following for 25.6 g of urea: a) the molar mass of urea? b) the number of moles of urea in 25.6 g urea? b) # of molecules of urea in 25.6 g of urea? c) # hydrogen ato ...
SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... Suggested Learning Activities Discuss and explain the particulate nature of matter. Use models or view computer simulations to discuss 1. the kinetic theory of matter 2 .the meaning of atoms, molecules and ions 3. conduct an activity to investigate diffusion of particles in solid, liquid and gas. Im ...
... Suggested Learning Activities Discuss and explain the particulate nature of matter. Use models or view computer simulations to discuss 1. the kinetic theory of matter 2 .the meaning of atoms, molecules and ions 3. conduct an activity to investigate diffusion of particles in solid, liquid and gas. Im ...
Chapter 3 PPt
... number and the same number of protons. Atoms do not necessarily have the same number of neutrons. • Atoms of the same element that have different numbers of neutrons are called isotopes. • One standard method of identifying isotopes is to write the mass number with a hyphen after the name of an elem ...
... number and the same number of protons. Atoms do not necessarily have the same number of neutrons. • Atoms of the same element that have different numbers of neutrons are called isotopes. • One standard method of identifying isotopes is to write the mass number with a hyphen after the name of an elem ...
goyal brothers prakashan
... Q.8. Explain with examples (i) atomic number (ii) mass number (iii) isotopes and (iv) isobars. Give any two uses of isotopes. Ans. (i) Atomic number : The number of protons in the nucleus of an atom is called its atomic number. Example : Chlorine has 17 protons in its nucleus, therefore, its atomic ...
... Q.8. Explain with examples (i) atomic number (ii) mass number (iii) isotopes and (iv) isobars. Give any two uses of isotopes. Ans. (i) Atomic number : The number of protons in the nucleus of an atom is called its atomic number. Example : Chlorine has 17 protons in its nucleus, therefore, its atomic ...
7.1 Describing Reactions
... of atoms on the left side does not equal the number of atoms on the right. The equation is not balanced. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. You can balance a chemical equation by changing the coefficients, the numbers that appear before t ...
... of atoms on the left side does not equal the number of atoms on the right. The equation is not balanced. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. You can balance a chemical equation by changing the coefficients, the numbers that appear before t ...
Section 1 Describing Chemical Reactions Chapter 8
... • The next step in writing a correct chemical equation is to replace the names of the reactants and products with appropriate symbols and formulas. • A formula equation represents the reactants and products of a chemical reaction by their symbols or formulas. • example: The formula equation for the ...
... • The next step in writing a correct chemical equation is to replace the names of the reactants and products with appropriate symbols and formulas. • A formula equation represents the reactants and products of a chemical reaction by their symbols or formulas. • example: The formula equation for the ...
EOCT Physical Science Study Guide August 2008
... information on study skills and general testtaking skills and strategies. It explains how to prepare before taking the test and what to do during the test to ensure the best test-taking situation possible. The TEST CONTENT section that begins on page 11 explains more specifically what the Physical S ...
... information on study skills and general testtaking skills and strategies. It explains how to prepare before taking the test and what to do during the test to ensure the best test-taking situation possible. The TEST CONTENT section that begins on page 11 explains more specifically what the Physical S ...
Answers - Pearson-Global
... Note: This is included because it is a simple example of a perfectly stable covalent compound where there aren’t four pairs of electrons around one of the atoms – in other words, it is nothing like a noble gas structure. Despite the impression often given at GCSE, such compounds are very common – al ...
... Note: This is included because it is a simple example of a perfectly stable covalent compound where there aren’t four pairs of electrons around one of the atoms – in other words, it is nothing like a noble gas structure. Despite the impression often given at GCSE, such compounds are very common – al ...
advanced placement chemistry workbook and note set
... because the formula of NaCl is just that – NaCl – there is no change in chemical nature if a “layer” of Na–Cl ions is removed – the sample is simply smaller (Figure 6). ...
... because the formula of NaCl is just that – NaCl – there is no change in chemical nature if a “layer” of Na–Cl ions is removed – the sample is simply smaller (Figure 6). ...
Stereochemistry - Kantipur Engineering College
... are mirror image but are superposible; therefore, are identical because of the plane of symmetry which dissects the molecule into two identical halves. Thus tartaric acid only has three stereoisomers. Compound V & VII and compound VI & VII are diastereomers because they are stereosiomers but not the ...
... are mirror image but are superposible; therefore, are identical because of the plane of symmetry which dissects the molecule into two identical halves. Thus tartaric acid only has three stereoisomers. Compound V & VII and compound VI & VII are diastereomers because they are stereosiomers but not the ...
Mole Concept - Shailendra Kumar Chemistry
... (c) 200 ml of 3.0 M NaCl is added to 300 ml of 4.0 M NaCl. (d) 200 ml of 2.0 M BaCl2 is added to 400 ml of 3.0 M BaCl2 and 400 ml of water. (e) 300 ml of 3.0 M NaCl is added to 200 ml of 4.0 M BaCl2. (f) 400 ml of 2.0 M HCl is added to 150 ml of 4.0 M NaOH. (g) 100 ml of 2.0 M HCl and 200 ml of 1.5 ...
... (c) 200 ml of 3.0 M NaCl is added to 300 ml of 4.0 M NaCl. (d) 200 ml of 2.0 M BaCl2 is added to 400 ml of 3.0 M BaCl2 and 400 ml of water. (e) 300 ml of 3.0 M NaCl is added to 200 ml of 4.0 M BaCl2. (f) 400 ml of 2.0 M HCl is added to 150 ml of 4.0 M NaOH. (g) 100 ml of 2.0 M HCl and 200 ml of 1.5 ...
Unit 2 Powerpoint Notes
... energy in the form of light and heat. • Products of combustion reactions are always carbon dioxide and water vapor. • Example: Combustion of propane C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) ...
... energy in the form of light and heat. • Products of combustion reactions are always carbon dioxide and water vapor. • Example: Combustion of propane C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) ...
Chemistry Essentials For Dummies
... Greenville, South Carolina. After a stint in the United States Army, he decided to try his hand at teaching. In 1971, he joined the chemistry faculty of Stephen F. Austin State University in Nacogdoches, Texas where he still teaches chemistry. In 1985, he started back to school part time and in 1991 ...
... Greenville, South Carolina. After a stint in the United States Army, he decided to try his hand at teaching. In 1971, he joined the chemistry faculty of Stephen F. Austin State University in Nacogdoches, Texas where he still teaches chemistry. In 1985, he started back to school part time and in 1991 ...
Chem Course Desc2. New
... 2) they know where to get the help, and 3) they act on that need to get the necessary help. This help may take the form of voluntary tutoring before or after school, assigned tutoring during school, or group study sessions. The vocabulary in chemistry is specific. For this reason, particular attent ...
... 2) they know where to get the help, and 3) they act on that need to get the necessary help. This help may take the form of voluntary tutoring before or after school, assigned tutoring during school, or group study sessions. The vocabulary in chemistry is specific. For this reason, particular attent ...
Chapter 3
... 1. Determine what reaction is occurring. What are the reactants, the products, and the physical states involved? 2. Write the unbalanced equation that summarizes the reaction described in step 1. 3. Balance the equation by inspection, starting with the most complicated molecule(s). The same number o ...
... 1. Determine what reaction is occurring. What are the reactants, the products, and the physical states involved? 2. Write the unbalanced equation that summarizes the reaction described in step 1. 3. Balance the equation by inspection, starting with the most complicated molecule(s). The same number o ...
Chapter 3: Mass Relationships in Chemical
... 58. A mass spectrometer works by ionizing atoms or molecules, and then accelerating them past oppositely charged plates. The mass is obtained by A) measuring the force of impact on a detecting screen, and then calculating the mass using force = mass acceleration. B) suspending the ions in an appli ...
... 58. A mass spectrometer works by ionizing atoms or molecules, and then accelerating them past oppositely charged plates. The mass is obtained by A) measuring the force of impact on a detecting screen, and then calculating the mass using force = mass acceleration. B) suspending the ions in an appli ...
chemistry writing team
... SOME BASIC CONCEPTS OF CHEMISTRY Law of conservation of mass : ‘Mass can neither be created nor destroyed.’ In all physical and chemical changes, the total mass of reactants is equal to that of products. Law of constant composition : A chemical compound is always found to be made of same elements co ...
... SOME BASIC CONCEPTS OF CHEMISTRY Law of conservation of mass : ‘Mass can neither be created nor destroyed.’ In all physical and chemical changes, the total mass of reactants is equal to that of products. Law of constant composition : A chemical compound is always found to be made of same elements co ...
Topic 1: Quantitative chemistry
... involving oxygen, that during any chemical reaction no atoms were destroyed. 2. Law of Definite Proportions: analytical tests showed that any compound consists of the same elements in definite proportions e.g. in every water molecule 88.88% of its mass is oxygen. 3. Law of Combining Volumes of Gases ...
... involving oxygen, that during any chemical reaction no atoms were destroyed. 2. Law of Definite Proportions: analytical tests showed that any compound consists of the same elements in definite proportions e.g. in every water molecule 88.88% of its mass is oxygen. 3. Law of Combining Volumes of Gases ...
Word - chemmybear.com
... B Proteins are long chains (polymers) of amino acids. Their properties depend on their shape. This shape comes from the order of the amino acids and the way the protein “folds up” as it is formed. Hydrogen bonding plays a huge role in how a protein folds up to give it a shape. When a protein is heat ...
... B Proteins are long chains (polymers) of amino acids. Their properties depend on their shape. This shape comes from the order of the amino acids and the way the protein “folds up” as it is formed. Hydrogen bonding plays a huge role in how a protein folds up to give it a shape. When a protein is heat ...
17.2 The Avogadro Number
... In single displacement reactions, one element is replaced by a similar element in a compound. The pattern for this replacement is easily predictable: if the element doing the replacing forms a positive ion, it replaces the element in the compound that forms a positive ion. If the substance doing the ...
... In single displacement reactions, one element is replaced by a similar element in a compound. The pattern for this replacement is easily predictable: if the element doing the replacing forms a positive ion, it replaces the element in the compound that forms a positive ion. If the substance doing the ...
Class 3 updated Sep 30 2011
... transported from the source onto the wafer surface and diffused into the wafer. The number of atoms that enter the wafer surface is limited by the solid solubility of the dopant in the wafer. The second step is drive-in, where the deposited wafer is heated in a diffusion furnace with an oxidizing or ...
... transported from the source onto the wafer surface and diffused into the wafer. The number of atoms that enter the wafer surface is limited by the solid solubility of the dopant in the wafer. The second step is drive-in, where the deposited wafer is heated in a diffusion furnace with an oxidizing or ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.