Brilliant Preparatory Section, Sitamarhi
... viii. All the reactants and products should be written as molecules including the elements like hydrogen, oxygen, nitrogen, fluorine chlorine, bromine and iodine as H2, O2, N2, F2, Cl2, Br2 and I2. ...
... viii. All the reactants and products should be written as molecules including the elements like hydrogen, oxygen, nitrogen, fluorine chlorine, bromine and iodine as H2, O2, N2, F2, Cl2, Br2 and I2. ...
Hydrocarbons and Fuels - Deans Community High School
... 7. The names, molecular and structural formula of alcohols (C1C8), straight and branched. 8. Carboxylic acids functional group COOH and properties of carboxylic acids 9. The names, molecular and structural formula of carboxylic acids (C1-C8), straight and branched. ...
... 7. The names, molecular and structural formula of alcohols (C1C8), straight and branched. 8. Carboxylic acids functional group COOH and properties of carboxylic acids 9. The names, molecular and structural formula of carboxylic acids (C1-C8), straight and branched. ...
Unit 5 Test Review
... Methane and oxygen are combined in a reaction vessel in amounts of 16 grams and 32 grams respectively. What amounts of reactants and products will be present in the reaction vessel once the reaction is complete? a. 0 grams CH4, 0 grams of O2, 44 grams of CO2, 36 grams of H2O b. 8 grams CH4, 0 grams ...
... Methane and oxygen are combined in a reaction vessel in amounts of 16 grams and 32 grams respectively. What amounts of reactants and products will be present in the reaction vessel once the reaction is complete? a. 0 grams CH4, 0 grams of O2, 44 grams of CO2, 36 grams of H2O b. 8 grams CH4, 0 grams ...
Document
... SORT The problem gives the mass of carbon dioxide and asks you to find the mass of glucose that can be produced. ...
... SORT The problem gives the mass of carbon dioxide and asks you to find the mass of glucose that can be produced. ...
Production of Materials by Jason Yu #2
... • The carbon-chain sections in cellulose could be changed to chemicals that at present mostly made from petroleum, if a chemical processes can be developed or micro-organisms found that can break the cellulose into glucose monomers, and glucose into three carbon-chains and four carbon-chains. • Thus ...
... • The carbon-chain sections in cellulose could be changed to chemicals that at present mostly made from petroleum, if a chemical processes can be developed or micro-organisms found that can break the cellulose into glucose monomers, and glucose into three carbon-chains and four carbon-chains. • Thus ...
Revisiting Molar Mass, Atomic Mass, and Mass
... Mass number is synonymous with the term nucleon number and refers to the total number of protons and neutrons in the nucleus of an atom. The symbol for the quantity is A and is dimensionless. The utility of this concept lies with providing scientists a way to distinguish between isotopes. The concep ...
... Mass number is synonymous with the term nucleon number and refers to the total number of protons and neutrons in the nucleus of an atom. The symbol for the quantity is A and is dimensionless. The utility of this concept lies with providing scientists a way to distinguish between isotopes. The concep ...
Objective: To state the assumptions of Dalton`s Atomic Theory
... Objective: To state the assumptions of Dalton's Atomic Theory. Explain the contributions of Dalton, Thompson, Rutherford to our understanding of the atom. Democritus 400 BC: atomos "indivisible" Dalton (early 1800's) Atomic Theory ...
... Objective: To state the assumptions of Dalton's Atomic Theory. Explain the contributions of Dalton, Thompson, Rutherford to our understanding of the atom. Democritus 400 BC: atomos "indivisible" Dalton (early 1800's) Atomic Theory ...
Chemical Reactions
... Word Equations • Word Equations: an equation in which the reactants and products in a chemical reaction are represented by words instead of chemical formulas. • The problem with word equations is they do not actually show the number of atoms or molecules of each substance… formulas would have to be ...
... Word Equations • Word Equations: an equation in which the reactants and products in a chemical reaction are represented by words instead of chemical formulas. • The problem with word equations is they do not actually show the number of atoms or molecules of each substance… formulas would have to be ...
Calculations and the Chemical Equation
... The Mole Concept and Atoms Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of pa ...
... The Mole Concept and Atoms Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of pa ...
File
... Using the tripeptide in (ii), state two types of bonding that can be formed and the groups in the tripeptide that are involved in this bonding. bond .................................................... groups ........................................................ bond ............................. ...
... Using the tripeptide in (ii), state two types of bonding that can be formed and the groups in the tripeptide that are involved in this bonding. bond .................................................... groups ........................................................ bond ............................. ...
Chemistry
... Materials and Technology Chemical research and development in the twentieth century have provided us with new materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such ...
... Materials and Technology Chemical research and development in the twentieth century have provided us with new materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such ...
CHEMISTRY Academic Standards Statement
... substances or to understand how substances are formed and removed in the environment. Chemistry is the science of analysing, transforming or manipulating substances and the molecular interpretation of the world around us. It is at the molecular level that major advances are made in many diverse area ...
... substances or to understand how substances are formed and removed in the environment. Chemistry is the science of analysing, transforming or manipulating substances and the molecular interpretation of the world around us. It is at the molecular level that major advances are made in many diverse area ...
Stoichiometry Objectives
... molecules, formula units, electrons, or ions. -called Avogadro’s number in honor of the Italian physicist and lawyer Amedeo Avogadro who, in 1811, determined the volume of one mole of a gas. -we round Avogadro’s number to three significant figures— 6.02 x 1023. - If you write out Avogadro’s number, ...
... molecules, formula units, electrons, or ions. -called Avogadro’s number in honor of the Italian physicist and lawyer Amedeo Avogadro who, in 1811, determined the volume of one mole of a gas. -we round Avogadro’s number to three significant figures— 6.02 x 1023. - If you write out Avogadro’s number, ...
Chapter K4
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
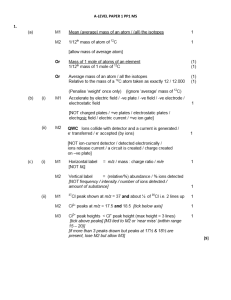
1 - A-Level Chemistry
... no lone / spare / non-bonded pair of electrons only score M2 if M1 correct or give ‘H’ in M1 ...
... no lone / spare / non-bonded pair of electrons only score M2 if M1 correct or give ‘H’ in M1 ...
Chapter K4
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
Chapter 11 - sacredheartphysicalscience
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
... Dalton’s Atomic Theory Based on Experiments • Dalton’s Theory John Dalton published his atomic theory in 1803. His theory stated that all substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed. Atoms of the same element are exactly alike, and atoms of d ...
Bis2A 06.Appendix A review of Red/Ox reactions
... red/ox states of atoms and simple molecules. In Bis2A, you will not need to balance equations or determine (calculate) the red/ox state of an atom or molecule. However, given a pair of compounds you will have to decide which one is the reduced form and which one is the oxidized form. This module may ...
... red/ox states of atoms and simple molecules. In Bis2A, you will not need to balance equations or determine (calculate) the red/ox state of an atom or molecule. However, given a pair of compounds you will have to decide which one is the reduced form and which one is the oxidized form. This module may ...
12 - einstein classes
... The reaction is reversible, and Le Chatelier’s principle suggests that a high pressure and low temperature are required to drive the reaction to the right, and thus form NH3. A low temperature gives a higher percentage conversion to NH3, but the reaction is slow in reaching equilibrium, and a cataly ...
... The reaction is reversible, and Le Chatelier’s principle suggests that a high pressure and low temperature are required to drive the reaction to the right, and thus form NH3. A low temperature gives a higher percentage conversion to NH3, but the reaction is slow in reaching equilibrium, and a cataly ...
Minimum electrophilicity principle in Lewis acid–base complexes of
... To reinvestigate the acidity strength of some boron trihalides (BX3; X = F, Cl and Br) from theoretical point of view, two sets of Lewis bases (weak and strong), which can form stable compounds with these acids, are considered here. It is expected that more stable complexes are formed by stronger ac ...
... To reinvestigate the acidity strength of some boron trihalides (BX3; X = F, Cl and Br) from theoretical point of view, two sets of Lewis bases (weak and strong), which can form stable compounds with these acids, are considered here. It is expected that more stable complexes are formed by stronger ac ...
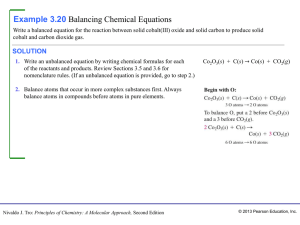
Chap 3 - HCC Learning Web
... Balancing equation is a very, very important question. To balance a chemical equation, you must make sure the number of atoms of each kind at both sides of the arrow is identical. Also start examining the most bulky species, that is, the one with the most different kinds of atoms and number of atoms ...
... Balancing equation is a very, very important question. To balance a chemical equation, you must make sure the number of atoms of each kind at both sides of the arrow is identical. Also start examining the most bulky species, that is, the one with the most different kinds of atoms and number of atoms ...
5 organic chemistry: functional groups
... There is no change in the number of valence electrons on any of the atoms in the reaction. Both before and after the reaction, each carbon atom shares a total of eight valence electrons and each hydrogen atom shares two electrons. Instead of electrons, the reaction involves the transfer of atoms—in ...
... There is no change in the number of valence electrons on any of the atoms in the reaction. Both before and after the reaction, each carbon atom shares a total of eight valence electrons and each hydrogen atom shares two electrons. Instead of electrons, the reaction involves the transfer of atoms—in ...
Avogadro`s Number, Moles and Molar Mass
... feasible. The volume that this many atoms or molecules would occupy is negligible. Reasonable masses of elements for scientists to work with are those that are based on the average atomic mass for an element. It was determined by the Italian scientist, Avogadro, that the number of atoms in exactly 1 ...
... feasible. The volume that this many atoms or molecules would occupy is negligible. Reasonable masses of elements for scientists to work with are those that are based on the average atomic mass for an element. It was determined by the Italian scientist, Avogadro, that the number of atoms in exactly 1 ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.