Chapter 19 Chemical Thermodynamics
... Translational: Movement of the entire molecule from one place to another. Vibrational: Periodic motion of atoms within a molecule. Rotational: Rotation of the molecule on about an axis or rotation about bonds. ...
... Translational: Movement of the entire molecule from one place to another. Vibrational: Periodic motion of atoms within a molecule. Rotational: Rotation of the molecule on about an axis or rotation about bonds. ...
Physical Chemistry 2.pdf
... nuclear chemistry. In solutions, we examine essentially the behaviour of homogenous mixtures involving pure substances. We shall also look at colloids, which differ from solutions only in terms of sizes of the solute. The topic of phase equilibrium looks at the physical transformation of pure substa ...
... nuclear chemistry. In solutions, we examine essentially the behaviour of homogenous mixtures involving pure substances. We shall also look at colloids, which differ from solutions only in terms of sizes of the solute. The topic of phase equilibrium looks at the physical transformation of pure substa ...
Chapter 3 Stoichiometry: Ratios of Combination
... • The data given allows an empirical formula determination with just a few more steps. • The mass of products (carbon dioxide and water) will be known so we work our way back. ...
... • The data given allows an empirical formula determination with just a few more steps. • The mass of products (carbon dioxide and water) will be known so we work our way back. ...
Chapter 2 - Atoms and the Periodic Table (test bank)
... In the early 1900s, Ernest Rutherford performed an experiment with thin foils of gold and alpha particles to probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have ...
... In the early 1900s, Ernest Rutherford performed an experiment with thin foils of gold and alpha particles to probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have ...
oxidation numbers
... 1 Work out the formula of the species before and after the change; 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 ...
... 1 Work out the formula of the species before and after the change; 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 ...
Chemistry 11 - Correspondence Studies
... of the element sodium, Na? In industry, when making extremely large amounts of chemicals through chemical reactions, how do chemists know how much of the reactants to react or how much product will be formed? This unit will answer these questions and other questions related to amount of matter. The ...
... of the element sodium, Na? In industry, when making extremely large amounts of chemicals through chemical reactions, how do chemists know how much of the reactants to react or how much product will be formed? This unit will answer these questions and other questions related to amount of matter. The ...
This question is about the elements in Period 3 of the Periodic Table
... The table below shows some successive ionisation energy data for atoms of three different elements X, Y and Z. Elements X, Y and Z are Ca, Sc and V but not in that order. ...
... The table below shows some successive ionisation energy data for atoms of three different elements X, Y and Z. Elements X, Y and Z are Ca, Sc and V but not in that order. ...
No Slide Title - MDC Faculty Home Pages
... Beyond the first 20 elements, the electron arrangements become more complicated and will not be covered in this course. ...
... Beyond the first 20 elements, the electron arrangements become more complicated and will not be covered in this course. ...
Introduction to chemistry Multiple Choice 1. Which SI prefix means
... 94. A solid with a specific gravity of 0.800 will float in water. Answer: True; Difficulty: medium; Reference: Section 2.9 95. The meter is a unit of length. Answer: True; Difficulty: easy; Reference: Section 2.7 96. The mass of a substance is independent of its location. Answer: True; Difficulty: e ...
... 94. A solid with a specific gravity of 0.800 will float in water. Answer: True; Difficulty: medium; Reference: Section 2.9 95. The meter is a unit of length. Answer: True; Difficulty: easy; Reference: Section 2.7 96. The mass of a substance is independent of its location. Answer: True; Difficulty: e ...
Stoichiometry: Calculations with Chemical Formulas and
... Calculating Empirical Formulas The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. ...
... Calculating Empirical Formulas The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. ...
5 pancakes 2 eggs
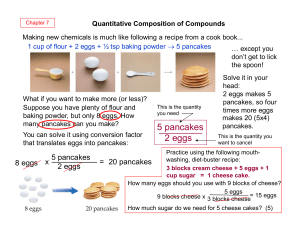
... that is used up during the reaction, i.e. limiting reactant. One bicycle needs 1 frame, 1 seat and 2 wheels, therefore not more than 3 bicycles can be made. The number of seats is the limiting part (reactant); one frame and two wheels are parts in excess; 3 bicycles is the yield. ...
... that is used up during the reaction, i.e. limiting reactant. One bicycle needs 1 frame, 1 seat and 2 wheels, therefore not more than 3 bicycles can be made. The number of seats is the limiting part (reactant); one frame and two wheels are parts in excess; 3 bicycles is the yield. ...
ppt
... First Law of Thermodynamics • You will recall from Chapter 5 that energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemica ...
... First Law of Thermodynamics • You will recall from Chapter 5 that energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemica ...
OXIDATION NUMBERS
... 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 If the charges on all the species (ions and electrons) on either si ...
... 2 If different numbers of the relevant species are on both sides, balance them 3 Work out the oxidation number of the element before and after the change 4 Add electrons to one side of the equation so the oxidation numbers balance 5 If the charges on all the species (ions and electrons) on either si ...
Slide 1
... I. Oxidation & Reduction -a substance which ________ oxidizes another substance by ________ accepting its ________ electrons is called an ________ oxidizing _____, agent which is also reduced the substance that is _______ -a substance which _______ reduces another substance by ______ losing ________ ...
... I. Oxidation & Reduction -a substance which ________ oxidizes another substance by ________ accepting its ________ electrons is called an ________ oxidizing _____, agent which is also reduced the substance that is _______ -a substance which _______ reduces another substance by ______ losing ________ ...
Chapter 6 Quantities in Chemical Reactions
... When the disengaged gasses are carefully examined, they are found to weigh 113.7 grs.; these are of two kinds, viz. 144 cubical inches of carbonic acid gas, weighing 100 grs. and 380 cubical inches of a very light gas, weighing only 13.7 grs.…and, when the water which has passed over into the bottle ...
... When the disengaged gasses are carefully examined, they are found to weigh 113.7 grs.; these are of two kinds, viz. 144 cubical inches of carbonic acid gas, weighing 100 grs. and 380 cubical inches of a very light gas, weighing only 13.7 grs.…and, when the water which has passed over into the bottle ...
11_20_12_BohrModel_Ions
... Infer how you think an S.W.B.A.T apply atom could become the bohr model to charged using your charged atoms knowledge of atomic (ions) structure from yesterday. **Get your agenda book out after you complete your Bell Ringer** ...
... Infer how you think an S.W.B.A.T apply atom could become the bohr model to charged using your charged atoms knowledge of atomic (ions) structure from yesterday. **Get your agenda book out after you complete your Bell Ringer** ...
Chemistry RTQ - Standardized Testing and Reporting (CA Dept of
... The following released test questions are taken from the Chemistry Standards Test. This test is one of the California Standards Tests administered as part of the Standardized Testing and Reporting (STAR) Program under policies set by the State Board of Education. All questions on the California Stan ...
... The following released test questions are taken from the Chemistry Standards Test. This test is one of the California Standards Tests administered as part of the Standardized Testing and Reporting (STAR) Program under policies set by the State Board of Education. All questions on the California Stan ...
Electron configuration
... being a source of energy, the fat is a source of water, because when it is used the reaction 2 C57H110O6(s) + 163 O2(g) 114 CO2(g) + 110 H2O(l) takes place. What mass of water can be made from 1.0 kg of fat? ...
... being a source of energy, the fat is a source of water, because when it is used the reaction 2 C57H110O6(s) + 163 O2(g) 114 CO2(g) + 110 H2O(l) takes place. What mass of water can be made from 1.0 kg of fat? ...
Welcome to AP Chemistry
... Rules for Determining Oxidation Number (this may be the one thing you did not learn in regular chemistry, but it’s very similar to charges, so it should be easy for you) Oxidation Number: A number assigned to an atom in a molecular compound or molecular ion that indicates the general distribution of ...
... Rules for Determining Oxidation Number (this may be the one thing you did not learn in regular chemistry, but it’s very similar to charges, so it should be easy for you) Oxidation Number: A number assigned to an atom in a molecular compound or molecular ion that indicates the general distribution of ...
Document
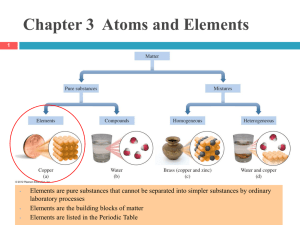
... #1. In terms of Particles An Element is made of atoms A Molecular compound (made of only nonmetals) is made up of molecules (Don’t forget the diatomic elements) Ionic Compounds (made of a metal and nonmetal parts) are made of formula units ...
... #1. In terms of Particles An Element is made of atoms A Molecular compound (made of only nonmetals) is made up of molecules (Don’t forget the diatomic elements) Ionic Compounds (made of a metal and nonmetal parts) are made of formula units ...
Welcome to AP Chemistry
... Rules for Determining Oxidation Number (this may be the one thing you did not learn in regular chemistry, but it’s very similar to charges, so it should be easy for you) Oxidation Number: A number assigned to an atom in a molecular compound or molecular ion that indicates the general distribution of ...
... Rules for Determining Oxidation Number (this may be the one thing you did not learn in regular chemistry, but it’s very similar to charges, so it should be easy for you) Oxidation Number: A number assigned to an atom in a molecular compound or molecular ion that indicates the general distribution of ...
Ch3
... • The data given allows an empirical formula determination with just a few more steps. • The mass of products (carbon dioxide and water) will be known so we work our way back. ...
... • The data given allows an empirical formula determination with just a few more steps. • The mass of products (carbon dioxide and water) will be known so we work our way back. ...
CHM203 - National Open University of Nigeria
... melting point, boiling point, solubility, etc., often give valuable clues about its structure. Conversely, if the structure of a compound is known, its physical properties can be predicated. The physical properties of a compound depend upon the number and nature of atoms constituting its structural ...
... melting point, boiling point, solubility, etc., often give valuable clues about its structure. Conversely, if the structure of a compound is known, its physical properties can be predicated. The physical properties of a compound depend upon the number and nature of atoms constituting its structural ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.