The Structure of the Atom
... An atom is the smallest particle of an element that still has the property of that element. ...
... An atom is the smallest particle of an element that still has the property of that element. ...
Basic Chem notes
... Lose an electron, become positive ion (ie. Na+1) Gain an electron, become negative ion (ie. Cl-1) IONS like to bond together, because OPPOSITES attract! ...
... Lose an electron, become positive ion (ie. Na+1) Gain an electron, become negative ion (ie. Cl-1) IONS like to bond together, because OPPOSITES attract! ...
CHEM 121 Chp 2 Spaulding
... Atoms of the same element that have a different number of neutrons ◦ The number of protons remains constant ...
... Atoms of the same element that have a different number of neutrons ◦ The number of protons remains constant ...
The Atom
... The ancient Greeks tried to explain matter, but the scientific study of the atom began with John Dalton in the early 1800s. K What I Know ...
... The ancient Greeks tried to explain matter, but the scientific study of the atom began with John Dalton in the early 1800s. K What I Know ...
Chapter 3 Lecture Notes
... Formula weight of the repeating unit (formula unit) is used for ionic substances. • Example: FW (NaCl) • = 23.0 amu + 35.5 amu = 58.5 amu. ...
... Formula weight of the repeating unit (formula unit) is used for ionic substances. • Example: FW (NaCl) • = 23.0 amu + 35.5 amu = 58.5 amu. ...
PowerPoint Presentation - The Atom: Chp 12 sect 2
... • The Greeks concluded that matter could be broken down into particles too small to be seen. • These particles were called atoms ...
... • The Greeks concluded that matter could be broken down into particles too small to be seen. • These particles were called atoms ...
Understanding the Atom
... correct if the statement is not correct. If the statement is not correct, change the underlined word(s) to make it correct. ...
... correct if the statement is not correct. If the statement is not correct, change the underlined word(s) to make it correct. ...
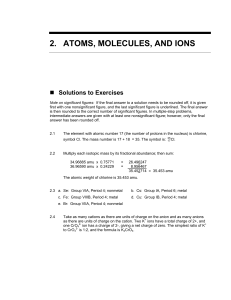
2.ATOMS, MOLECULES, AND IONS
... b. You recognize the fact that whenever a cation can have multiple oxidation states (1+, 2+, and 5+ in this case) the name of the compound must indicate the charge. Therefore, the names of the compounds in part (a) would be exy(I) sulfate, exy(II) sulfate, and exy(V) sulfate, respectively. 2.26 a. T ...
... b. You recognize the fact that whenever a cation can have multiple oxidation states (1+, 2+, and 5+ in this case) the name of the compound must indicate the charge. Therefore, the names of the compounds in part (a) would be exy(I) sulfate, exy(II) sulfate, and exy(V) sulfate, respectively. 2.26 a. T ...
Chem midterm review powerpoint
... • Round the answer to the same number of decimal places as the original measurement with the fewest decimal places. Multiplication and division • Round the answer to the same number of significant figures as the original measurement with the fewest significant figures. ...
... • Round the answer to the same number of decimal places as the original measurement with the fewest decimal places. Multiplication and division • Round the answer to the same number of significant figures as the original measurement with the fewest significant figures. ...
Document
... 47) Applying Concepts: The elements shown in the diagram are ______. a) part of the same group b) part of the same period c) not related ...
... 47) Applying Concepts: The elements shown in the diagram are ______. a) part of the same group b) part of the same period c) not related ...
C1403_Final Exam p. 1 Friday, January 23, 2004 Printed Last Name
... UNDER NO CIRCUMSTANCES are you to make marks on the bubble sheet except in the appropriate bubbles for marking an answer you believe to be correct. You may write on the exam sheets themselves for the purpose of doing calculations. A PERIODIC TABLE OF THE ELEMENTS can be found at the end of this exam ...
... UNDER NO CIRCUMSTANCES are you to make marks on the bubble sheet except in the appropriate bubbles for marking an answer you believe to be correct. You may write on the exam sheets themselves for the purpose of doing calculations. A PERIODIC TABLE OF THE ELEMENTS can be found at the end of this exam ...
Understanding Atomic Structure of an Element
... What makes up the Elements of an Atom • An element is a material that cannot be broken down into any simpler substance • Thanks to Dalton, we know that an element is made up of Atoms that are all identical in mass and no two elements have the same mass • Then Thompson and Borh discovered subatomic ...
... What makes up the Elements of an Atom • An element is a material that cannot be broken down into any simpler substance • Thanks to Dalton, we know that an element is made up of Atoms that are all identical in mass and no two elements have the same mass • Then Thompson and Borh discovered subatomic ...
Regents questions
... Arranging the elements by atomic weight leads to an order slightly different from that in a modern periodic table, where the arrangement is by atomic number. Why does this happen? ...
... Arranging the elements by atomic weight leads to an order slightly different from that in a modern periodic table, where the arrangement is by atomic number. Why does this happen? ...
File
... Directions: This packet will serve as your notes for this chapter. Follow along with the PowerPoint presentation and fill in the missing information. Important terms / ideas are in all capitals and bolded! ...
... Directions: This packet will serve as your notes for this chapter. Follow along with the PowerPoint presentation and fill in the missing information. Important terms / ideas are in all capitals and bolded! ...
https://www.ted.com/talks/just_how_small_is_an_atom#
... • An electron is never found between energy levels. Instead, it “jumps” from one level to the next. ...
... • An electron is never found between energy levels. Instead, it “jumps” from one level to the next. ...
Introducing the Atom - Core Concepts: Periodic Table
... 1. Explain that radioactive decay is a random process, like flipping a coin. Ask students: a. What does random mean? b. What are the odds of getting heads on any flip of a coin? 2. Explain that radioactive decay is measured by half-life. Explain that radioactive isotopes decay by losing half of ...
... 1. Explain that radioactive decay is a random process, like flipping a coin. Ask students: a. What does random mean? b. What are the odds of getting heads on any flip of a coin? 2. Explain that radioactive decay is measured by half-life. Explain that radioactive isotopes decay by losing half of ...
Element
... isotopic mass = 62.94 amu) and copper-65 (30.83%; isotopic mass = 64.93 amu). Calculate the atomic mass of copper and check the answer in the ...
... isotopic mass = 62.94 amu) and copper-65 (30.83%; isotopic mass = 64.93 amu). Calculate the atomic mass of copper and check the answer in the ...
Atomic Systems and Bonding
... Bonding Energy, the Curve Shape, and Bonding Type Properties depend on shape, bonding type and values of curves: they vary for different materials. Bonding energy (minimum on curve) is the energy that would be required to separate the two atoms to an infinite separation. Modulus of elasticity ...
... Bonding Energy, the Curve Shape, and Bonding Type Properties depend on shape, bonding type and values of curves: they vary for different materials. Bonding energy (minimum on curve) is the energy that would be required to separate the two atoms to an infinite separation. Modulus of elasticity ...
Chemistry Definitions
... levels. The orbital with the lowest energy is always filled first 12. Hund’s Rule of Multiplicity: When filling subshells that contain more than one orbital with the same energy level, each orbital must be singly occupied before electrons are paired 13. Pauli’s Exclusion Principle: An orbital cannot ...
... levels. The orbital with the lowest energy is always filled first 12. Hund’s Rule of Multiplicity: When filling subshells that contain more than one orbital with the same energy level, each orbital must be singly occupied before electrons are paired 13. Pauli’s Exclusion Principle: An orbital cannot ...
File
... 2. Make a hypothesis: What will happen when an electric current is passed through water? ...
... 2. Make a hypothesis: What will happen when an electric current is passed through water? ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.