Chapter 4
... Ancient Greek Theory • Democritus named atoms, for the Greek word which means indivisible. • Aristotle believed that all substances were built from four elements—earth, air, fire and water. • This four were combinations of four qualities—hot, cold, dry and wet as shown. ...
... Ancient Greek Theory • Democritus named atoms, for the Greek word which means indivisible. • Aristotle believed that all substances were built from four elements—earth, air, fire and water. • This four were combinations of four qualities—hot, cold, dry and wet as shown. ...
Chapter 3
... Nitrous oxide (N2O) is also called “laughing gas.” It can be prepared by the thermal decomposition of ammonium nitrate (NH4NO3). The other product is H2O. The balanced equation for this reaction is: NH4NO3 N2O + 2H2O How many grams of N2O are formed if 0.46 mole of NH4NO3 is used in the reaction? A) ...
... Nitrous oxide (N2O) is also called “laughing gas.” It can be prepared by the thermal decomposition of ammonium nitrate (NH4NO3). The other product is H2O. The balanced equation for this reaction is: NH4NO3 N2O + 2H2O How many grams of N2O are formed if 0.46 mole of NH4NO3 is used in the reaction? A) ...
Chapter 04 Atomic Theory Notes
... Ancient Greek Theory • Democritus named atoms, for the Greek word which means indivisible. • Aristotle believed that all substances were built from four elements—earth, air, fire and water. • This four were combinations of four qualities—hot, cold, dry and wet as shown. ...
... Ancient Greek Theory • Democritus named atoms, for the Greek word which means indivisible. • Aristotle believed that all substances were built from four elements—earth, air, fire and water. • This four were combinations of four qualities—hot, cold, dry and wet as shown. ...
Slide 1
... – Dry air contains about 0.002% Ne – That’s 500,000,000,000,000,000 atoms of neon every breath ...
... – Dry air contains about 0.002% Ne – That’s 500,000,000,000,000,000 atoms of neon every breath ...
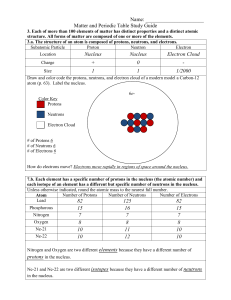
Matter and the Periodic Table Study Guide Answer Key
... 3.b. Compounds are formed by combining two or more different elements and compounds have properties that are different from their constituent elements. 3.f. Use the periodic table to identify elements in simple compounds. Compound List the number of each type of atom making up the compound NH4 1 Nit ...
... 3.b. Compounds are formed by combining two or more different elements and compounds have properties that are different from their constituent elements. 3.f. Use the periodic table to identify elements in simple compounds. Compound List the number of each type of atom making up the compound NH4 1 Nit ...
Revision topic 1-3
... All gases respond in a similar way to changes in temperature, pressure and volume. They exert a pressure, that depends on the amount of gas and the temperature There is no bonding between molecules The molecules may move in all directions allowing the gas to expand throughout any container ...
... All gases respond in a similar way to changes in temperature, pressure and volume. They exert a pressure, that depends on the amount of gas and the temperature There is no bonding between molecules The molecules may move in all directions allowing the gas to expand throughout any container ...
Standard Atomic Notation Standard Atomic Notation
... • Although they exist, we will not draw elements with more than three orbits. Extra Rules: • You have to put electrons into the lowest orbits first. • Put electrons in the second and third orbits one at a time until you get 4 electrons in the orbit, and then start to pair them up. Draw the Bohr-Ruth ...
... • Although they exist, we will not draw elements with more than three orbits. Extra Rules: • You have to put electrons into the lowest orbits first. • Put electrons in the second and third orbits one at a time until you get 4 electrons in the orbit, and then start to pair them up. Draw the Bohr-Ruth ...
Word Equations • a summary
... In any chemical reaction, atoms are neither created nor destroyed, just rearranged. Therefore, because of the conservation of mass, chemical equations are balanced when the number of each type of atom is the same on either side of the arrow sign. ...
... In any chemical reaction, atoms are neither created nor destroyed, just rearranged. Therefore, because of the conservation of mass, chemical equations are balanced when the number of each type of atom is the same on either side of the arrow sign. ...
Unit 2 Notes Name - Mr. Walsh`s AP Chemistry
... o Ionic compounds are soluble in water if the sum of all of their attractions to the water molecules is greater than their attraction to each other. A good rule of thumb (though there are exceptions) is that almost all compounds with alkali metal and halogen ions are soluble. Most (but not all) comp ...
... o Ionic compounds are soluble in water if the sum of all of their attractions to the water molecules is greater than their attraction to each other. A good rule of thumb (though there are exceptions) is that almost all compounds with alkali metal and halogen ions are soluble. Most (but not all) comp ...
Chapter 2
... INTRODUCTION The history of the modern atomic theory spans nearly 2000 years. However, it is only in the last 200 years that there has been a good understanding of the atom and even still new discoveries about microscopic atomic structure are being made. It is important as a scientist to know the pe ...
... INTRODUCTION The history of the modern atomic theory spans nearly 2000 years. However, it is only in the last 200 years that there has been a good understanding of the atom and even still new discoveries about microscopic atomic structure are being made. It is important as a scientist to know the pe ...
Matter - TeacherWeb
... Soft, brittle, and dull in appearance Poor conductors of heat or electricity Can share electrons or gain 1, 2, or 3 electrons to form negative ions ...
... Soft, brittle, and dull in appearance Poor conductors of heat or electricity Can share electrons or gain 1, 2, or 3 electrons to form negative ions ...
CH 101 Study Guide Test 2
... Identify Avogadro’s number and convert from moles to atoms, and from atoms to moles Convert from grams to moles and moles to grams calculate formula weight What is molar mass (same as formula weight) and identify its units (g/mol) Calculate the mass percent of an element in a compound ...
... Identify Avogadro’s number and convert from moles to atoms, and from atoms to moles Convert from grams to moles and moles to grams calculate formula weight What is molar mass (same as formula weight) and identify its units (g/mol) Calculate the mass percent of an element in a compound ...
Name Period _____ Table _____ Vocabulary Log: ATOMS
... Tell the difference between chemical and physical changes ...
... Tell the difference between chemical and physical changes ...
Name: _ Date: Period: ______ Page: ______ Atomic Structure and
... Rutherford performed another group of experiments similar to the first using nitrogen as the target to confirm his model of the atom. Most of the alpha particles went straight through the empty space of the nitrogen atoms; however, a few bumped into the nucleus and bounced off. In addition, he disco ...
... Rutherford performed another group of experiments similar to the first using nitrogen as the target to confirm his model of the atom. Most of the alpha particles went straight through the empty space of the nitrogen atoms; however, a few bumped into the nucleus and bounced off. In addition, he disco ...
I. Atoms II. Chemical Symbols III. Structure
... The top number here (for oxygen 8) is the element’s atomic number. This is the number of protons in the element; therefore every atom of oxygen has 8 protons in its nucleus. Each specific element has a specific number of protons. If an atom has the same number of protons as another, the two are the ...
... The top number here (for oxygen 8) is the element’s atomic number. This is the number of protons in the element; therefore every atom of oxygen has 8 protons in its nucleus. Each specific element has a specific number of protons. If an atom has the same number of protons as another, the two are the ...
History of the Atom
... When a small paddle wheel was placed in the path of the cathode rays the wheel was set in motion. ...
... When a small paddle wheel was placed in the path of the cathode rays the wheel was set in motion. ...
A Few Laws • Conservation of Matter-For any
... • In chemistry, we deal with matter on the large scale • Dalton’s Law tells us we need to measure elements in some manner that can be looked at as an “atomic measure” • If all apples weighed 23.0ounces and all oranges weighed 13.2ounces, I can readily scale these up to any other weight and be assure ...
... • In chemistry, we deal with matter on the large scale • Dalton’s Law tells us we need to measure elements in some manner that can be looked at as an “atomic measure” • If all apples weighed 23.0ounces and all oranges weighed 13.2ounces, I can readily scale these up to any other weight and be assure ...
What is the smallest particle of an element that retains the properties
... 55. When elements in the s and p blocks form ions by gaining or losing electrons, they attain the same electron configuration as the nearest… 59. Magnetic metals have unpaired electrons. Which of the following metals would be least magnetic? a) K b) Zn c) Na d) Mn 60. What would have to happen in or ...
... 55. When elements in the s and p blocks form ions by gaining or losing electrons, they attain the same electron configuration as the nearest… 59. Magnetic metals have unpaired electrons. Which of the following metals would be least magnetic? a) K b) Zn c) Na d) Mn 60. What would have to happen in or ...
No Slide Title
... Although Silicon is the second most abundant element in the earth’s crust, it never occurs in nature alone as an element. Instead it occurs in the form of Silica, which is a combination of Silicon and different elements. This Silica compound must be processed to yield Silicon that is 99.999999 ...
... Although Silicon is the second most abundant element in the earth’s crust, it never occurs in nature alone as an element. Instead it occurs in the form of Silica, which is a combination of Silicon and different elements. This Silica compound must be processed to yield Silicon that is 99.999999 ...
Unit 2 -- Atomic Structure, Periodic Table, and
... diagrams that show the valence electrons as dots. Since only the valence electrons are involved in bonding, this is very useful in predicting which atoms will combine chemically. ...
... diagrams that show the valence electrons as dots. Since only the valence electrons are involved in bonding, this is very useful in predicting which atoms will combine chemically. ...
Communicating Research to the General Public
... transition metals. Transition metals, highlighted in red in Figure 1.1 comprise a majority of the periodic table and can be considered the building blocks of inorganic chemistry just as carbon is considered the building block of organic chemistry. We are familiar with many of the transition metals b ...
... transition metals. Transition metals, highlighted in red in Figure 1.1 comprise a majority of the periodic table and can be considered the building blocks of inorganic chemistry just as carbon is considered the building block of organic chemistry. We are familiar with many of the transition metals b ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.