Chapter 4 powerpoint
... • The atomic number of an atom is given by its number of protons. The mass number of an atom is the sum of its neutrons and protons. atomic number = number of protons = number of electrons mass number = atomic number + number of neutrons ...
... • The atomic number of an atom is given by its number of protons. The mass number of an atom is the sum of its neutrons and protons. atomic number = number of protons = number of electrons mass number = atomic number + number of neutrons ...
Curriculum Plan
... Distinguish between ionic and covalent chemical bonding, 5.7.A.4 Identify general properties of ionic compounds and of 5.7.A.7 covalent compounds, Use electron dot diagrams to represent ionic and covalent bonding in formula units and molecules, Recognize types of ions: cation and anion, monatomic an ...
... Distinguish between ionic and covalent chemical bonding, 5.7.A.4 Identify general properties of ionic compounds and of 5.7.A.7 covalent compounds, Use electron dot diagrams to represent ionic and covalent bonding in formula units and molecules, Recognize types of ions: cation and anion, monatomic an ...
atomic number on the periodic table
... • Dmitri Mendeleev, a Russian scientist born in Siberia in 1834, is known as the father of the periodic table of the elements • The periodic table is designed to help you predict chemical and physical properties of elements ...
... • Dmitri Mendeleev, a Russian scientist born in Siberia in 1834, is known as the father of the periodic table of the elements • The periodic table is designed to help you predict chemical and physical properties of elements ...
Chem EOC Review Cumulative Free Response
... a) A 7.0 liter balloon at room temperature (22oC) contains hydrogen gas. If the balloon is carried outside to where the temperature is –3.0oC, what volume will the balloon occupy? b) A 5.0 liter tank of oxygen gas is at a pressure of 3 atm. What volume of oxygen will be available if the oxygen is us ...
... a) A 7.0 liter balloon at room temperature (22oC) contains hydrogen gas. If the balloon is carried outside to where the temperature is –3.0oC, what volume will the balloon occupy? b) A 5.0 liter tank of oxygen gas is at a pressure of 3 atm. What volume of oxygen will be available if the oxygen is us ...
Chapter 4
... ____ 50. Which of the following equals one atomic mass unit? a. the mass of one electron b. the mass of one helium-4 atom c. the mass of one carbon-12 atom d. one-twelfth the mass of one carbon-12 atom ____ 51. Which of the following statements is NOT true? a. Protons have a positive charge. b. Elec ...
... ____ 50. Which of the following equals one atomic mass unit? a. the mass of one electron b. the mass of one helium-4 atom c. the mass of one carbon-12 atom d. one-twelfth the mass of one carbon-12 atom ____ 51. Which of the following statements is NOT true? a. Protons have a positive charge. b. Elec ...
Balance this equation:
... CO reacting to form iron and carbon dioxide. Which of the following is the correct full balanced chemical equation for the reaction depicted? ...
... CO reacting to form iron and carbon dioxide. Which of the following is the correct full balanced chemical equation for the reaction depicted? ...
Review Jeopardy
... b.) Atoms of different elements combine in simple whole-number ratios to form compounds ...
... b.) Atoms of different elements combine in simple whole-number ratios to form compounds ...
Atoms and Elements: Are they Related?
... atomic #. • How many protons does Oxygen have? What is Oxygen’s atomic #? • Neon has 10 protons. What is its atomic #? • If an atom has 35 protons, what is the name of the atom? ...
... atomic #. • How many protons does Oxygen have? What is Oxygen’s atomic #? • Neon has 10 protons. What is its atomic #? • If an atom has 35 protons, what is the name of the atom? ...
PUC Schools - cloudfront.net
... from liquid to gas, the process is a) Exothermic b) Endothermic c) Neutral d) Kinetic 28. (7.c) The temperature of iced water melting is _____ oC. The temperature of boiling water is _____ oC. a) 100, 200 b) 0, 100 c) 100, 0 d) None 29. (7.b) If in a chemical reaction, the ...
... from liquid to gas, the process is a) Exothermic b) Endothermic c) Neutral d) Kinetic 28. (7.c) The temperature of iced water melting is _____ oC. The temperature of boiling water is _____ oC. a) 100, 200 b) 0, 100 c) 100, 0 d) None 29. (7.b) If in a chemical reaction, the ...
6-2 Notes: The Atom
... The charges or protons and electrons are opposite but _________, so the charges cancel out. If the numbers of electrons and protons become unequal, the atom becomes a charged particle called an ______. An atom that loses one or more electrons becomes a _______________ charged ion. An atom that gains ...
... The charges or protons and electrons are opposite but _________, so the charges cancel out. If the numbers of electrons and protons become unequal, the atom becomes a charged particle called an ______. An atom that loses one or more electrons becomes a _______________ charged ion. An atom that gains ...
Atomic Structure Worksheet
... Use the name examples below to help you name the atoms and ions. See if you can figure out the name differences between cation names and anion names or use your textbook for further help. Use the information given on table below to calculate atomic mass. If not enough information is given on the tab ...
... Use the name examples below to help you name the atoms and ions. See if you can figure out the name differences between cation names and anion names or use your textbook for further help. Use the information given on table below to calculate atomic mass. If not enough information is given on the tab ...
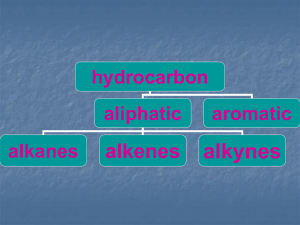
Methane - ARZELORIVAS IS
... This reaction has the following characteristic properties. It doesn't take place in the dark or at low temperatures. It occurs in the presence of ultraviolet light or at temperatures above 250oC. Once the reaction gets started, it continues after the light is turned off. The products of the ...
... This reaction has the following characteristic properties. It doesn't take place in the dark or at low temperatures. It occurs in the presence of ultraviolet light or at temperatures above 250oC. Once the reaction gets started, it continues after the light is turned off. The products of the ...
Atoms - Chemistry R: 4(AE)
... •If an atom receives, energy, the atom becomes excited and electrons jump to higher energy levels. •EXCITED STATE: an atom with higher potential energy than in the ground state because electrons have “jumped” to a higher energy level. ...
... •If an atom receives, energy, the atom becomes excited and electrons jump to higher energy levels. •EXCITED STATE: an atom with higher potential energy than in the ground state because electrons have “jumped” to a higher energy level. ...
SCH4U - Unit 1
... space where the electron is MOST LIKELY to be found, however we will not focus on the mathematical in this course, Regions where electrons are most likely to be found are called orbitals. For every value of n, there are n types of orbitals and n2 actual orbitals 1st energy level has 1 type of orbita ...
... space where the electron is MOST LIKELY to be found, however we will not focus on the mathematical in this course, Regions where electrons are most likely to be found are called orbitals. For every value of n, there are n types of orbitals and n2 actual orbitals 1st energy level has 1 type of orbita ...
FREE Sample Here
... Full file at http://emailtestbank.com/ Test-Bank-for-Campbell-Biology-with-MasteringBiology-9th-Edition-by-Reece 24) What is the maximum number of electrons in a single 2 p orbital of an atom? A) 1 B) 2 C) 3 D) 4 E) 5 Answer: B Topic: Concept 2.2 Skill: Knowledge/Comprehension 25) The organic molec ...
... Full file at http://emailtestbank.com/ Test-Bank-for-Campbell-Biology-with-MasteringBiology-9th-Edition-by-Reece 24) What is the maximum number of electrons in a single 2 p orbital of an atom? A) 1 B) 2 C) 3 D) 4 E) 5 Answer: B Topic: Concept 2.2 Skill: Knowledge/Comprehension 25) The organic molec ...
PowerPoint - De Anza College
... A. Isotopes, Atomic Number, and Mass Number Isotopes are atoms of the same element that have a different number of neutrons. the number of protons (Z) ...
... A. Isotopes, Atomic Number, and Mass Number Isotopes are atoms of the same element that have a different number of neutrons. the number of protons (Z) ...
Chapter 2 - WordPress.com
... A. Isotopes, Atomic Number, and Mass Number Isotopes are atoms of the same element that have a different number of neutrons. the number of protons (Z) ...
... A. Isotopes, Atomic Number, and Mass Number Isotopes are atoms of the same element that have a different number of neutrons. the number of protons (Z) ...
04_Lecture Atoms and Elements
... atomic number. • Columns of elements have similar properties and are called groups or families. • Elements on the left side are metals. They tend to lose electrons in their chemical changes. • Elements on the upper right side are nonmetals. They tend to gain electrons in their chemical changes. • El ...
... atomic number. • Columns of elements have similar properties and are called groups or families. • Elements on the left side are metals. They tend to lose electrons in their chemical changes. • Elements on the upper right side are nonmetals. They tend to gain electrons in their chemical changes. • El ...
04_Lecture Atoms and Elements
... • There are about 91 different elements in nature, and consequently about 91 different kinds of atoms. • Scientists have succeeded in making about 20 synthetic elements (not found in nature). • The exact number of naturally occurring elements is controversial because some elements previously conside ...
... • There are about 91 different elements in nature, and consequently about 91 different kinds of atoms. • Scientists have succeeded in making about 20 synthetic elements (not found in nature). • The exact number of naturally occurring elements is controversial because some elements previously conside ...
Elements of Chemical Structure and Inorganic Nomenclature
... separated matter by all the methods (chemical and physical) available to them until they could not separate it any further. They felt this separation must result in the building block of matter, which they called the atom (from the Greek word for indivisible). They also observed that the basic units ...
... separated matter by all the methods (chemical and physical) available to them until they could not separate it any further. They felt this separation must result in the building block of matter, which they called the atom (from the Greek word for indivisible). They also observed that the basic units ...
Lesson Plan - cloudfront.net
... Democritus wondered if matter could be divided infinitely. He believed that eventually you couldn’t split matter into smaller pieces. He said if he took a loaf of bread and kept breaking it in half, at some point you would get to a piece so small that it would be impossible to break. He came up with ...
... Democritus wondered if matter could be divided infinitely. He believed that eventually you couldn’t split matter into smaller pieces. He said if he took a loaf of bread and kept breaking it in half, at some point you would get to a piece so small that it would be impossible to break. He came up with ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood (Pages 735
... *4. As the totally _________ water is heated by the surrounding ___ __ of the room, its temperature will _____ continue to ________, and as more ____________ is added the temperature of the water will rise _________ 0o C and continue rising until it reaches ___ __ causing __________________, a speci ...
... *4. As the totally _________ water is heated by the surrounding ___ __ of the room, its temperature will _____ continue to ________, and as more ____________ is added the temperature of the water will rise _________ 0o C and continue rising until it reaches ___ __ causing __________________, a speci ...
No Slide Title
... How many H atoms are in 72.5 g of C3H8O ? 1 mol C3H8O = (3 x 12) + (8 x 1) + 16 = ______ g C3H8O 1 mol C3H8O molecules = ___________ mol H atoms 1 mol H = ___________ atoms H 1 mol C3H8O 8 mol H atoms 6.022 x 1023 H atoms 72.5 g C3H8O x ...
... How many H atoms are in 72.5 g of C3H8O ? 1 mol C3H8O = (3 x 12) + (8 x 1) + 16 = ______ g C3H8O 1 mol C3H8O molecules = ___________ mol H atoms 1 mol H = ___________ atoms H 1 mol C3H8O 8 mol H atoms 6.022 x 1023 H atoms 72.5 g C3H8O x ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.