The nucleus - VCE Chemistry
... • In 1913 Soddy explained these observations by introducing the idea of isotopes (from the Greek, meaning 'same place') as elements with the same chemical properties but containing atoms which differed in mass, physical properties and radioactive behaviour. • The relative atomic mass of such an elem ...
... • In 1913 Soddy explained these observations by introducing the idea of isotopes (from the Greek, meaning 'same place') as elements with the same chemical properties but containing atoms which differed in mass, physical properties and radioactive behaviour. • The relative atomic mass of such an elem ...
Mass Number, A
... 5. In a chemical reac2on, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements. Yes, except __________________________________ ___ ...
... 5. In a chemical reac2on, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements. Yes, except __________________________________ ___ ...
Terms Used in Part 3
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Atomic mass: the average mass number of all isotopes of an atom. Round this number to find the mass number of the atom. Isotope: Atom with the same number of protons but a different number of neutrons an ...
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Atomic mass: the average mass number of all isotopes of an atom. Round this number to find the mass number of the atom. Isotope: Atom with the same number of protons but a different number of neutrons an ...
Slide 1
... The new philosophical idea of what is a fundamental particle at an atomic scale required new phenomenological ideas (such as ‘matter waves’) along with new mathematical ideas (such as ‘traveling wave packets’). Where did Bohr get his new philosophical ideas for modeling the atom? ...
... The new philosophical idea of what is a fundamental particle at an atomic scale required new phenomenological ideas (such as ‘matter waves’) along with new mathematical ideas (such as ‘traveling wave packets’). Where did Bohr get his new philosophical ideas for modeling the atom? ...
Chapter # 4 notes
... An element is a fundamental or elementary substance that cannot be broken down into simpler substances by chemical means. Each element has a number. Beginning with hydrogen as 1, the elements are numbered in order of increasing complexity. Most substances can be decomposed into two or more simpler s ...
... An element is a fundamental or elementary substance that cannot be broken down into simpler substances by chemical means. Each element has a number. Beginning with hydrogen as 1, the elements are numbered in order of increasing complexity. Most substances can be decomposed into two or more simpler s ...
PS 2.3
... an advertisement poster on its everyday use. You want to make this poster as appealing as possible for your immediate classmates and school community, so that people will take the time to read and learn about the everyday use of several elements found on the Periodic Table Your poster needs to inclu ...
... an advertisement poster on its everyday use. You want to make this poster as appealing as possible for your immediate classmates and school community, so that people will take the time to read and learn about the everyday use of several elements found on the Periodic Table Your poster needs to inclu ...
Ch 04 AtomicStructure
... C. Atoms contain a tiny, positively charged nucleus. D. Atoms that combine do so in simple whole-number ratios. Which of the following was originally a tenet of Dalton's atomic theory, but had to be revised about a century ago? A. Atoms are tiny indivisible particles. B. The atoms of any one element ...
... C. Atoms contain a tiny, positively charged nucleus. D. Atoms that combine do so in simple whole-number ratios. Which of the following was originally a tenet of Dalton's atomic theory, but had to be revised about a century ago? A. Atoms are tiny indivisible particles. B. The atoms of any one element ...
mass number - KCPE-KCSE
... Mass spectrometry is an accurate instrumental technique used to determine the relative isotopic mass (mass of each individual isotope relative to carbon-12) and the relative abundance for each isotope. From this, the relative atomic mass of the element can be calculated. Some uses of mass spectromet ...
... Mass spectrometry is an accurate instrumental technique used to determine the relative isotopic mass (mass of each individual isotope relative to carbon-12) and the relative abundance for each isotope. From this, the relative atomic mass of the element can be calculated. Some uses of mass spectromet ...
Chemistry - Schoodoodle
... word atoms today. Many contemporaries of Leucippus and Democritus, including Plato and Aristotle, did not accept the idea that matter was made up of particles that have distinct properties of their own. Instead, they believed that all matter was uniform in composition, no matter how small the piece ...
... word atoms today. Many contemporaries of Leucippus and Democritus, including Plato and Aristotle, did not accept the idea that matter was made up of particles that have distinct properties of their own. Instead, they believed that all matter was uniform in composition, no matter how small the piece ...
8.3 Metals - UNSW Chemistry
... This calculated atomic weight (80.8) is not a bad estimate of the experimentally determined atomic weight (79.9). The chemistry of bromine will be similar to the chemistry of chlorine and iodine. An enormous number of possible chemical reactions could be discussed. For example: all three elements fo ...
... This calculated atomic weight (80.8) is not a bad estimate of the experimentally determined atomic weight (79.9). The chemistry of bromine will be similar to the chemistry of chlorine and iodine. An enormous number of possible chemical reactions could be discussed. For example: all three elements fo ...
Chemical Quantities PPT
... Calculate the formula mass of calcium chloride Write the formula from the name given Ca2+ (from group II) and Cl- (from group VII) Formula is CaCl2 due to charge balance Formula mass: Sum of the atomic masses of atoms in the formula (1 Ca atom + 2 Cl atoms) 40.08 amu = 40.08 amu 1 Ca atom 35.45 amu ...
... Calculate the formula mass of calcium chloride Write the formula from the name given Ca2+ (from group II) and Cl- (from group VII) Formula is CaCl2 due to charge balance Formula mass: Sum of the atomic masses of atoms in the formula (1 Ca atom + 2 Cl atoms) 40.08 amu = 40.08 amu 1 Ca atom 35.45 amu ...
1 - kurtniedenzu
... b. Stephen Jay Gould c. Throckmorton P. Guildersleeve d. Ernest B. Rutherford 15. Which numbered arrow in the diagram below gives the best indicator of the time at which the particle model of the atom became generally accepted by chemists and physicists? ...
... b. Stephen Jay Gould c. Throckmorton P. Guildersleeve d. Ernest B. Rutherford 15. Which numbered arrow in the diagram below gives the best indicator of the time at which the particle model of the atom became generally accepted by chemists and physicists? ...
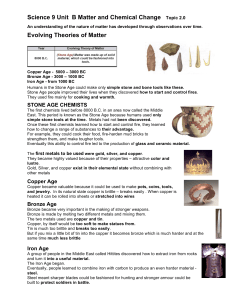
An understanding of the nature of matter has developed
... graphite. Or it can be a hard, clear substance called diamond. This later became a problem, when more elements were discovered, because they ran out of planets. John Dalton developed a new set of symbols in the early 1800’s to improve communication between chemists. ...
... graphite. Or it can be a hard, clear substance called diamond. This later became a problem, when more elements were discovered, because they ran out of planets. John Dalton developed a new set of symbols in the early 1800’s to improve communication between chemists. ...
Molar Mass - Science With Horne
... Molar Mass = the mass of 1 mole of a substance in grams (also known as Gram Formula Weight or Formula Weight) mass given on periodic table is in amu Because amu and grams are relative we can say the masses on the periodic table are in grams To find molar mass add all the atomic masses of the element ...
... Molar Mass = the mass of 1 mole of a substance in grams (also known as Gram Formula Weight or Formula Weight) mass given on periodic table is in amu Because amu and grams are relative we can say the masses on the periodic table are in grams To find molar mass add all the atomic masses of the element ...
Name: Date: ______ Period: _____ Chemistry 1st semester final
... 37. How are the elements arranged on the periodic table?By increasing atomic number 38. Why do element in the same family have similar chemical properties?b/c they have the same number of valance electrons 39. What type of elements is found in the upper right hand part of the periodic table?nonmetal ...
... 37. How are the elements arranged on the periodic table?By increasing atomic number 38. Why do element in the same family have similar chemical properties?b/c they have the same number of valance electrons 39. What type of elements is found in the upper right hand part of the periodic table?nonmetal ...
Thomson`s Model of the Atom
... Ancient Greek Models of Atoms Aristotle thought that all substances were made of only four elements—earth, air, fire, and water. He did not think there was a limit to the division of matter. For many centuries, most people accepted Aristotle’s views on the structure of matter. By the 1800s, scienti ...
... Ancient Greek Models of Atoms Aristotle thought that all substances were made of only four elements—earth, air, fire, and water. He did not think there was a limit to the division of matter. For many centuries, most people accepted Aristotle’s views on the structure of matter. By the 1800s, scienti ...
Chapter 2. Atoms, Molecules, and Ions - College Test bank
... • Goal: find the charge on the electron to determine its mass. • Oil drops are sprayed above a positively charged plate containing a small hole. • As the oil drops fall through the hole they acquire a negative charge. • Gravity forces the drops downward. The applied electric field forces the drops u ...
... • Goal: find the charge on the electron to determine its mass. • Oil drops are sprayed above a positively charged plate containing a small hole. • As the oil drops fall through the hole they acquire a negative charge. • Gravity forces the drops downward. The applied electric field forces the drops u ...
Hewitt/Lyons/Suchocki/Yeh, Conceptual Integrated Science
... “All things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another.” —Richard Feynman ...
... “All things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another.” —Richard Feynman ...
Notebook - Science
... covalent bond: involves the sharing of electron pairs between atoms where the electron pairs are known as shared pairs or bonding pairs single bond: two atoms are held together by one electron pair double bond: bonds formed when two atoms share two or more pairs of elctrons triple bond: when two ato ...
... covalent bond: involves the sharing of electron pairs between atoms where the electron pairs are known as shared pairs or bonding pairs single bond: two atoms are held together by one electron pair double bond: bonds formed when two atoms share two or more pairs of elctrons triple bond: when two ato ...
Stoichometry Notes (Unit 2)
... Checking the equation, we find that we have two sodium atoms, one sulfur atom, four oxygen atoms, one barium atom and two chlorine atoms on the reactant side of the equation and one barium atom, one sulfur atom, four oxygen atoms, two sodium atoms, and two chlorine atoms on the product side. Thus t ...
... Checking the equation, we find that we have two sodium atoms, one sulfur atom, four oxygen atoms, one barium atom and two chlorine atoms on the reactant side of the equation and one barium atom, one sulfur atom, four oxygen atoms, two sodium atoms, and two chlorine atoms on the product side. Thus t ...
Metals
... Relative molecular mass Relative atomic mass (Ar) is used to describe the mass of atoms. Relative molecular mass (Mr) is used to describe the mass of molecules. (Mr) = the mass of a molecule of a substance or compound relative to the mass of an atom of the ...
... Relative molecular mass Relative atomic mass (Ar) is used to describe the mass of atoms. Relative molecular mass (Mr) is used to describe the mass of molecules. (Mr) = the mass of a molecule of a substance or compound relative to the mass of an atom of the ...
CHEMISTry is life - World of Teaching
... -Too often kids get to high school chemistry and they are scared before they even begin. -My goal is to shape a positive image in their minds about chemistry so that they can be more prepared mentally for high school. -I will do this by showing them how applicable chemistry is to every day life. It ...
... -Too often kids get to high school chemistry and they are scared before they even begin. -My goal is to shape a positive image in their minds about chemistry so that they can be more prepared mentally for high school. -I will do this by showing them how applicable chemistry is to every day life. It ...
Chapter 4 and 25 Study Guide
... 16. What is the relative mass of protons, neutrons, and electrons? Protons 1; Neutrons 1; electrons about zero 17. Are atoms positive, negative, or neutral? How does the number of protons relate to the number of electrons? Atoms are neutral: number of protons and electrons in atoms are always equal ...
... 16. What is the relative mass of protons, neutrons, and electrons? Protons 1; Neutrons 1; electrons about zero 17. Are atoms positive, negative, or neutral? How does the number of protons relate to the number of electrons? Atoms are neutral: number of protons and electrons in atoms are always equal ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.