Name___________________________________ Physical
... 8) A term that means "without water" is ________________________. 9) How can you drive the water out of a hydrate? By ________________________. _________ _________ 10) Which of the following correctly shows the formula for a hydrate? A) MgSO4 (H2 O)7 B) H2 O C) H2 O2 ...
... 8) A term that means "without water" is ________________________. 9) How can you drive the water out of a hydrate? By ________________________. _________ _________ 10) Which of the following correctly shows the formula for a hydrate? A) MgSO4 (H2 O)7 B) H2 O C) H2 O2 ...
Unit 3 Practice Test
... A. Non-metals generally have the higher electronegativities and tend to attract electrons to themselves in a chemical bond. B. Elements with high ionization energies tend to have small atomic radii. C. Elements with high electronegativities generally form ions with small radii. D. The second ionizat ...
... A. Non-metals generally have the higher electronegativities and tend to attract electrons to themselves in a chemical bond. B. Elements with high ionization energies tend to have small atomic radii. C. Elements with high electronegativities generally form ions with small radii. D. The second ionizat ...
The chemical elements are fundamental building materials of matter
... • 1.A: All matter is made of atoms. There are a limited number of types of atoms: these are the elements. • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are orga ...
... • 1.A: All matter is made of atoms. There are a limited number of types of atoms: these are the elements. • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are orga ...
Chemistry 2011-2012
... SC1 Students will analyze the nature of matter and its classifications. SC1a. Relate the role of nuclear fusion in producing essentially all elements heavier than helium. SC1b. Identify substances based on chemical and physical properties. SC2 Students will relate how the Law of Conservation of Matt ...
... SC1 Students will analyze the nature of matter and its classifications. SC1a. Relate the role of nuclear fusion in producing essentially all elements heavier than helium. SC1b. Identify substances based on chemical and physical properties. SC2 Students will relate how the Law of Conservation of Matt ...
4 - College of Arts and Sciences
... Formula Of Reactants and Products • IONIC – ONLY Ions Are Shown • NET IONIC – SPECTATOR Ions Are Removed From Ionic Equation • SPECTATOR Ions – Ions that do not take part in the chemical reaction ...
... Formula Of Reactants and Products • IONIC – ONLY Ions Are Shown • NET IONIC – SPECTATOR Ions Are Removed From Ionic Equation • SPECTATOR Ions – Ions that do not take part in the chemical reaction ...
Lecture 2
... 5:00 PM for dinner and instructions.) If you are not a judge, you may attend the Science Expo and write a detailed summary of at least two projects. Either is extra-credit points or a lab make-up. ...
... 5:00 PM for dinner and instructions.) If you are not a judge, you may attend the Science Expo and write a detailed summary of at least two projects. Either is extra-credit points or a lab make-up. ...
CP-Chem Ch 3 PowerPoint(Atomic Theory
... Section 3.2 Review 1) Why was it important for the Cathode Ray to pass through a vacuum tube? 2) Compare the 3 sub-atomic particles in terms of location in the atom, mass, and charge. (you may use a diagram in your response) 3) Describe one conclusion made by each of the following scientists that l ...
... Section 3.2 Review 1) Why was it important for the Cathode Ray to pass through a vacuum tube? 2) Compare the 3 sub-atomic particles in terms of location in the atom, mass, and charge. (you may use a diagram in your response) 3) Describe one conclusion made by each of the following scientists that l ...
Common Chemical Formula List
... Chemical Formula Definition: An expression which states the number and type of atoms present in a molecule of a substance. Chemical formulas such as HClO4 can be divided into empirical formula, molecular formula, and structural formula. Chemical symbols of elements in the chemical formula represent ...
... Chemical Formula Definition: An expression which states the number and type of atoms present in a molecule of a substance. Chemical formulas such as HClO4 can be divided into empirical formula, molecular formula, and structural formula. Chemical symbols of elements in the chemical formula represent ...
semester two final review key units 5 and 6 only
... Bases: ionic compounds that break apart to form a negatively charged hydroxide ion (OH-) in water Neutral: A solution that has a pH of 7. It is neither acidic nor basic Amphoteric: a substance that can act as an acid or base under various conditions pH scale: a measure of the strength of acidity or ...
... Bases: ionic compounds that break apart to form a negatively charged hydroxide ion (OH-) in water Neutral: A solution that has a pH of 7. It is neither acidic nor basic Amphoteric: a substance that can act as an acid or base under various conditions pH scale: a measure of the strength of acidity or ...
Numerical Methods
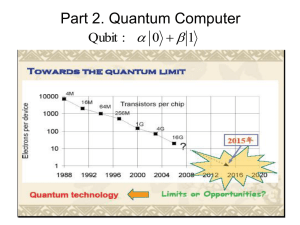
... *. use quantum mechanical phenomena ( such as superposition and entanglement ) to perform operations on data ...
... *. use quantum mechanical phenomena ( such as superposition and entanglement ) to perform operations on data ...
4 - College of Arts and Sciences
... Empirical / Molecular Formulas Determine the empirical formula of a compound that has (by mass) 21.7% C ; 9.6% O ; and 68.7% F. • answer C3OF6 Determine the molecular formula of the compound if its molar mass = 166 • Answer: same as empirical formula ...
... Empirical / Molecular Formulas Determine the empirical formula of a compound that has (by mass) 21.7% C ; 9.6% O ; and 68.7% F. • answer C3OF6 Determine the molecular formula of the compound if its molar mass = 166 • Answer: same as empirical formula ...
Class notes on Work Potential Energy and
... Remember: if is 1800 then cos = -1 If is 900 then cos = 0 Work is scalar and can be positive or negative. Work is measured in newton meters or joules. Energy is the capacity for doing work, which is inherent to an object or physical system. Energy is measured in joules and can neither be cre ...
... Remember: if is 1800 then cos = -1 If is 900 then cos = 0 Work is scalar and can be positive or negative. Work is measured in newton meters or joules. Energy is the capacity for doing work, which is inherent to an object or physical system. Energy is measured in joules and can neither be cre ...
Chapter 3
... 1 molecule N2 = 3 molecules H2 1 molecule N2 = 2 molecules NH3 3 molecules H2 = 2 molecules NH3 LEP #7 ...
... 1 molecule N2 = 3 molecules H2 1 molecule N2 = 2 molecules NH3 3 molecules H2 = 2 molecules NH3 LEP #7 ...
Eighth Grade Review - PAMS-Doyle
... • pH is a measure of the hydrogen ion concentration in a solution. The pH scale ranges from 0–14. Solutions with a pH lower than 7 are acidic; solutions with a pH greater than 7 are basic. A pH of 7 is neutral. ...
... • pH is a measure of the hydrogen ion concentration in a solution. The pH scale ranges from 0–14. Solutions with a pH lower than 7 are acidic; solutions with a pH greater than 7 are basic. A pH of 7 is neutral. ...
Chemistry at Karlsruhe 1860
... Atomic Weights (Relative and Equivalent only) The Periodic Table Diatomic Nature of Elemental Gases, O2, H2 Whether Atoms are Real or just a useful idea Relative weights of Carbon and Oxygen Formulas of water and carbon oxides Concept of Covalent Bonding ...
... Atomic Weights (Relative and Equivalent only) The Periodic Table Diatomic Nature of Elemental Gases, O2, H2 Whether Atoms are Real or just a useful idea Relative weights of Carbon and Oxygen Formulas of water and carbon oxides Concept of Covalent Bonding ...
Chemical Formulas and Chemical Compounds
... A correctly written chemical formula must represent the known facts about the analytically determined composition of the compound Used to represent amounts of substance -H2O can represent: 1 mole of water molecules 1 molecule of water 1 molar mass of water molecules Monatomic Ions: Ions formed from ...
... A correctly written chemical formula must represent the known facts about the analytically determined composition of the compound Used to represent amounts of substance -H2O can represent: 1 mole of water molecules 1 molecule of water 1 molar mass of water molecules Monatomic Ions: Ions formed from ...
PAP Chemistry - Fall Final Review
... 11. What is Avogadro’s Number? 6.02 X 1023 12. How many atoms are in 1 mole of each element? 6.02 X 1023 Does this number change? No, since the equation is 1 mole = 6.02 X 1023 atoms 13. Be able to do mol-mass conversions 14. Determine the number of protons, electrons, and neutrons in the following ...
... 11. What is Avogadro’s Number? 6.02 X 1023 12. How many atoms are in 1 mole of each element? 6.02 X 1023 Does this number change? No, since the equation is 1 mole = 6.02 X 1023 atoms 13. Be able to do mol-mass conversions 14. Determine the number of protons, electrons, and neutrons in the following ...
Document
... In a chemical equation, like the one below, you will notice that there are regular sized numbers in front of some of the molecules and small numbers after certain atoms within a molecule. The little number is called the subscript and tells how many of a certain type of atom are in a molecule. The bi ...
... In a chemical equation, like the one below, you will notice that there are regular sized numbers in front of some of the molecules and small numbers after certain atoms within a molecule. The little number is called the subscript and tells how many of a certain type of atom are in a molecule. The bi ...
Things to Know to Pass the Chemistry Regents
... 70. Pressure and volume indirect, P up, V down (PVC pipe) 71. Temperature and pressure direct, T up, P up 72. Temperature and volume direct, T up, V up 73. Gases most ideal at high temp and low pressure (have more energy and free to spread out) *ideal is summer vacation 74. He and H most ideal becau ...
... 70. Pressure and volume indirect, P up, V down (PVC pipe) 71. Temperature and pressure direct, T up, P up 72. Temperature and volume direct, T up, V up 73. Gases most ideal at high temp and low pressure (have more energy and free to spread out) *ideal is summer vacation 74. He and H most ideal becau ...