QUIZ: History of Atomic Structure
... D) The cathode ray tube proved that electrons have a negative charge. 4. Which was used to determine the value for charge of the electron? A) the gold foil experiment B) deflection of cathode rays by electric and magnetic fields C) The oil drop experiment D) the periodic table E) the mass spectromet ...
... D) The cathode ray tube proved that electrons have a negative charge. 4. Which was used to determine the value for charge of the electron? A) the gold foil experiment B) deflection of cathode rays by electric and magnetic fields C) The oil drop experiment D) the periodic table E) the mass spectromet ...
Chapter 2 PowerPoint
... • Atoms of a single element that possess different numbers of neutrons • Radioactive isotopes are unstable and emit radiation as the nucleus breaks up – Half-life – time it takes for one-half of the atoms in a sample to decay ...
... • Atoms of a single element that possess different numbers of neutrons • Radioactive isotopes are unstable and emit radiation as the nucleus breaks up – Half-life – time it takes for one-half of the atoms in a sample to decay ...
chapt02_lecture from text
... • Atoms of a single element that possess different numbers of neutrons • Radioactive isotopes are unstable and emit radiation as the nucleus breaks up – Half-life – time it takes for one-half of the atoms in a sample to decay ...
... • Atoms of a single element that possess different numbers of neutrons • Radioactive isotopes are unstable and emit radiation as the nucleus breaks up – Half-life – time it takes for one-half of the atoms in a sample to decay ...
Unit 2: Biochem Notes
... and basic solutions more acidic. Reactions occur best at a specific pH. - Humans must maintain a blood pH of 7.4 (+ or - .5). If the pH changes more than .5, then many chemical reactions in our cells would be negatively affected. Disease, possibly death, will occur if blood pH homeostasis is not mai ...
... and basic solutions more acidic. Reactions occur best at a specific pH. - Humans must maintain a blood pH of 7.4 (+ or - .5). If the pH changes more than .5, then many chemical reactions in our cells would be negatively affected. Disease, possibly death, will occur if blood pH homeostasis is not mai ...
Chemistry--Chapter 5: Atomic Structure and the Periodic Table
... rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction. B. Just How Small Is an Atom? 1. Unimaginably small! 2. A pure copper penny would contain 2.4 x 1022 atoms, while there are only 6 x 109 people on earth; a 1-cm line of copp ...
... rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction. B. Just How Small Is an Atom? 1. Unimaginably small! 2. A pure copper penny would contain 2.4 x 1022 atoms, while there are only 6 x 109 people on earth; a 1-cm line of copp ...
CHAPTER 2 ATOMS, MOLECULES, AND IONS 1 CHAPTER TWO
... d. Water (H2O) is always 1 g hydrogen for every 8 g of O present, while H2O2 is always 1 g hydrogen for every 16 g of O present. These are distinctly different compounds, each with its own unique relative number and types of atoms present. e. A chemical equation involves a reorganization of the atom ...
... d. Water (H2O) is always 1 g hydrogen for every 8 g of O present, while H2O2 is always 1 g hydrogen for every 16 g of O present. These are distinctly different compounds, each with its own unique relative number and types of atoms present. e. A chemical equation involves a reorganization of the atom ...
Elements
... Chemical formulas – atoms are indicated by the element symbols; number of each atom is indicated by a subscript – a number that appears to the right of and below the symbol for the element ...
... Chemical formulas – atoms are indicated by the element symbols; number of each atom is indicated by a subscript – a number that appears to the right of and below the symbol for the element ...
ROTATING COORDINATE SYSTEMS - FacStaff Home Page for CBU
... These are two coupled differential equations (both y and vy in the ax equation, and both x and vx in the ay equation). The methods to solve these are not easily identified. However, there is more than one way to attack a problem. We can also try using Conservation of Energy! As viewed from the rotat ...
... These are two coupled differential equations (both y and vy in the ax equation, and both x and vx in the ay equation). The methods to solve these are not easily identified. However, there is more than one way to attack a problem. We can also try using Conservation of Energy! As viewed from the rotat ...
CVB101 – Lecture 3 Chemical Bonding • Chemical bonding
... The maximum amount of solute that will dissolve in a given quantity of solvent (at a specific temperature) Some compounds are very soluble e.g. NaCl o It is possible to make very concentrated solutions on NaCl Other compounds are not very soluble e.g. AgCl o If AgCl solid is placed in water, o ...
... The maximum amount of solute that will dissolve in a given quantity of solvent (at a specific temperature) Some compounds are very soluble e.g. NaCl o It is possible to make very concentrated solutions on NaCl Other compounds are not very soluble e.g. AgCl o If AgCl solid is placed in water, o ...
fo-Balancing Chemical Notes
... In a chemical reaction, atoms are NOT created or destroyed. What changes in a chemical reaction is the connections (bonds) between atoms. In the first reaction shown above, two hydrogen atoms (H) start out bonded to each other. During the course of the chemical reaction, this H-H bond breaks and a n ...
... In a chemical reaction, atoms are NOT created or destroyed. What changes in a chemical reaction is the connections (bonds) between atoms. In the first reaction shown above, two hydrogen atoms (H) start out bonded to each other. During the course of the chemical reaction, this H-H bond breaks and a n ...
Nature of Atoms Atomic Structure Atomic number Atomic mass
... ◦ Cl atom gains an electron to become Cl– ◦ Opposite charges attract so that Na+ and Cl– remain associated as an ionic compound ...
... ◦ Cl atom gains an electron to become Cl– ◦ Opposite charges attract so that Na+ and Cl– remain associated as an ionic compound ...
Fall Exam 4 - Chemistry - University of Kentucky
... The total number of molecular orbitals formed does not always equal the number of atomic orbitals combined. In H2 molecules, the two 1s orbitals combine constructively, which results in one bonding orbital and one nonbonding orbital Electrons placed in antibonding orbitals stabilize the species. Whe ...
... The total number of molecular orbitals formed does not always equal the number of atomic orbitals combined. In H2 molecules, the two 1s orbitals combine constructively, which results in one bonding orbital and one nonbonding orbital Electrons placed in antibonding orbitals stabilize the species. Whe ...
Atomic Structure
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
Chapter 10: Simple Harmonic Motion
... potential difference of 1 V Also, we see from the work integral form of V, that [V]=[N/C][m] or [N/C]=[V/m] So, electric field is the “rate” of change of the electric potential with position ...
... potential difference of 1 V Also, we see from the work integral form of V, that [V]=[N/C][m] or [N/C]=[V/m] So, electric field is the “rate” of change of the electric potential with position ...
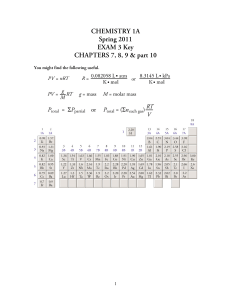
Exam 3 Key
... 4. A(n) unsaturated fat is a triglyceride that has one or more carboncarbon double bonds. 5. Hydrogenation is a process by which hydrogen is added to an unsaturated triglyceride to convert double bonds to single bonds. This can be done by combining the unsaturated triglyceride with hydrogen gas and ...
... 4. A(n) unsaturated fat is a triglyceride that has one or more carboncarbon double bonds. 5. Hydrogenation is a process by which hydrogen is added to an unsaturated triglyceride to convert double bonds to single bonds. This can be done by combining the unsaturated triglyceride with hydrogen gas and ...
Investigating Matter Notes
... that can be ________________ or ________________. Characteristics that can be observed are called __________________ properties. Characteristics that can be measured are called __________________ properties. The set of properties that a particular substance has is unique. No other substance has that ...
... that can be ________________ or ________________. Characteristics that can be observed are called __________________ properties. Characteristics that can be measured are called __________________ properties. The set of properties that a particular substance has is unique. No other substance has that ...
Chemistry - Napa Valley College
... Most of the strongest bonds in organisms are covalent bonds that form a cell’s molecules Weak chemical bonds are also indispensable Many large biological molecules are held in their functional form by weak bonds The reversibility of weak bonds can be an ...
... Most of the strongest bonds in organisms are covalent bonds that form a cell’s molecules Weak chemical bonds are also indispensable Many large biological molecules are held in their functional form by weak bonds The reversibility of weak bonds can be an ...
Unit 3 - Chemistry
... a uniform pudding of positives charge with enough negative electrons scattered within to counterbalance that positive charge. ...
... a uniform pudding of positives charge with enough negative electrons scattered within to counterbalance that positive charge. ...
e c n i
... atoms are present in this molecule b. W hen no subscript number is shown: it is understood that there is only one atom present: H2O = 2 Hydrogen atoms and only one Oxygen atom are present in this molecule ...
... atoms are present in this molecule b. W hen no subscript number is shown: it is understood that there is only one atom present: H2O = 2 Hydrogen atoms and only one Oxygen atom are present in this molecule ...
Physical Science
... If elements in brackets or parenthesis, treat same as in math. Coefficients multiple the entire molecule atoms You must add all reactant molecules together & compare w/ all molecules in the products ...
... If elements in brackets or parenthesis, treat same as in math. Coefficients multiple the entire molecule atoms You must add all reactant molecules together & compare w/ all molecules in the products ...
File
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found ...
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found ...
Chapter 2
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found ...
... • Key to the chemical behavior of an atom lies in the number and arrangement of its electrons in their orbitals • Bohr model – electrons in discrete orbits • Modern physics defines orbital as area around a nucleus where an electron is most likely to be found ...