Stoichiometry - Taylor County Schools
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
Stoichiometry - coercingmolecules
... of sodium ascorbate are present? c. How many moles of C are present? d. How many moles of Na are present? e. How many formula units of sodium ascorbate are present? f. How many atoms of Na are present? ...
... of sodium ascorbate are present? c. How many moles of C are present? d. How many moles of Na are present? e. How many formula units of sodium ascorbate are present? f. How many atoms of Na are present? ...
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
Answers
... e) None of these is a displacement reaction The reactions above have been labeled for you. ...
... e) None of these is a displacement reaction The reactions above have been labeled for you. ...
CYPRUS
... Course lectures are in Greek and the students must take a final exam for each course. The final grade results from a combination of homework grades, intermediate (mid-term) exams, literature projects or laboratory reports. There are no prerequisite courses, but in a series of related courses (e.g., ...
... Course lectures are in Greek and the students must take a final exam for each course. The final grade results from a combination of homework grades, intermediate (mid-term) exams, literature projects or laboratory reports. There are no prerequisite courses, but in a series of related courses (e.g., ...
Novel Methods and Materials in Development of Liquid Carrier
... Back in 1999, my alternative civilian service approaching its end, I was looking for a job. For some innate reason I was focusing pharmaceutical industry only. I have to admit that at that time I already had a very ambitious wish to continue my education carrier within the frame of PhD studies in th ...
... Back in 1999, my alternative civilian service approaching its end, I was looking for a job. For some innate reason I was focusing pharmaceutical industry only. I have to admit that at that time I already had a very ambitious wish to continue my education carrier within the frame of PhD studies in th ...
Document
... Suppose you want to ‘whip’ a batch of hydrogen iodide, following the balanced chemical equation: ...
... Suppose you want to ‘whip’ a batch of hydrogen iodide, following the balanced chemical equation: ...
Stoichiometry - Mr Field's Chemistry Class
... It is found by titration that 25.0 cm3 of an unknown solution of sulfuric acid is just neutralised by adding 11.3 cm3 of1.00 mol dm-3 sodium hydroxide. What is the concentration of sulfuric acid in the sample. H2SO4 + 2 NaOH Na2SO4 + 2 H2O ...
... It is found by titration that 25.0 cm3 of an unknown solution of sulfuric acid is just neutralised by adding 11.3 cm3 of1.00 mol dm-3 sodium hydroxide. What is the concentration of sulfuric acid in the sample. H2SO4 + 2 NaOH Na2SO4 + 2 H2O ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
... • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
Worked solutions to the problems
... our warnings cannot be comprehensive - your students will still need your careful supervision. We have also not included specific details for handling or disposal of the products of these lab exercises, as these will vary greatly from country to country, but we know that you will employ best-practic ...
... our warnings cannot be comprehensive - your students will still need your careful supervision. We have also not included specific details for handling or disposal of the products of these lab exercises, as these will vary greatly from country to country, but we know that you will employ best-practic ...
Chem Course Desc2. New
... 3.2 Demonstrate the ability to differentiate between ionic and covalent compounds. Be able to name these compounds. Relate electronegativity and ionization energy to bond formation. ( C.S. 2.g ) 3.3 Demonstrate that chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and ...
... 3.2 Demonstrate the ability to differentiate between ionic and covalent compounds. Be able to name these compounds. Relate electronegativity and ionization energy to bond formation. ( C.S. 2.g ) 3.3 Demonstrate that chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and ...
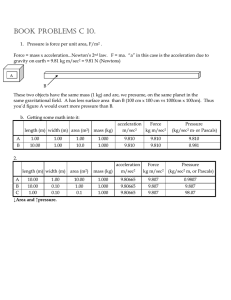
book problems c 10.
... atomic mass, and the unit cell length, determined from x-ray methods. To be useful for this purpose, the crystal must be free of defects. Very accurate values of these quantities for silicon have been measured at the National Institute for Standards and Technology (NIST). To use this approach, it is ...
... atomic mass, and the unit cell length, determined from x-ray methods. To be useful for this purpose, the crystal must be free of defects. Very accurate values of these quantities for silicon have been measured at the National Institute for Standards and Technology (NIST). To use this approach, it is ...
mclintock.ch6 [Compatibility Mode]
... ► Acid–base neutralization reactions are processes in which H+ ions from an acid react with OH- ions from a base to yield water. An ionic compound called a salt is also produced. The “salt” produced need not be common table salt. Any ionic compound produced in an acid–base reaction is called a salt. ...
... ► Acid–base neutralization reactions are processes in which H+ ions from an acid react with OH- ions from a base to yield water. An ionic compound called a salt is also produced. The “salt” produced need not be common table salt. Any ionic compound produced in an acid–base reaction is called a salt. ...
Chemistry
... aspects of general chemistry. Chemistry is mastered when students make the right connections in three key areas: topics that are related, conceptual reasoning with quantitative work, and the different modes of communicating information. McMurry/Fay’s Chemistry, Sixth Edition breaks through the tradi ...
... aspects of general chemistry. Chemistry is mastered when students make the right connections in three key areas: topics that are related, conceptual reasoning with quantitative work, and the different modes of communicating information. McMurry/Fay’s Chemistry, Sixth Edition breaks through the tradi ...
Mixed-space approach for calculation of vibration
... For an ionic crystal, when the periodic supercell approach is employed, one has to distinguish two cases when calculating phonon frequencies: (i) at the nonzero qS , the lattice vibration does not result in macroscopic electric fields; and (ii) Away from the qS , the lattice vibration results in macr ...
... For an ionic crystal, when the periodic supercell approach is employed, one has to distinguish two cases when calculating phonon frequencies: (i) at the nonzero qS , the lattice vibration does not result in macroscopic electric fields; and (ii) Away from the qS , the lattice vibration results in macr ...
Calculations with Chemical Formulas and Equations
... The trick: • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... The trick: • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Chapter 3 2013
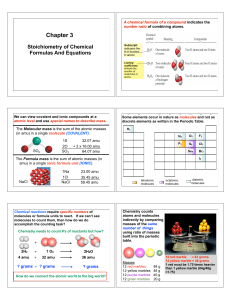
... There are many conversion factors in a formula. 1 molecule H2O = 1 atom O and 2 atoms of H Molecular mass H2O = (2 x 1.008)+15.99 = 18.00 amu Molar mass = 18.00 g/mol H2O 1 mole H2O = 6.022 x 1023 molecules H2O 1 mole H2O = 2 mol H atoms = 2 x 6.02 x 1023 H atoms 1 mole H2O = 1 mol O atoms = 6.02 x ...
... There are many conversion factors in a formula. 1 molecule H2O = 1 atom O and 2 atoms of H Molecular mass H2O = (2 x 1.008)+15.99 = 18.00 amu Molar mass = 18.00 g/mol H2O 1 mole H2O = 6.022 x 1023 molecules H2O 1 mole H2O = 2 mol H atoms = 2 x 6.02 x 1023 H atoms 1 mole H2O = 1 mol O atoms = 6.02 x ...
B.Sc. (Hons.) Chemistry
... collision frequency; collision diameter; mean free path and viscosity of gases, including their temperature and pressure dependence, relation between mean free path and coefficient of viscosity, calculation of σ from η; variation of viscosity with temperature and pressure. Maxwell distribution and i ...
... collision frequency; collision diameter; mean free path and viscosity of gases, including their temperature and pressure dependence, relation between mean free path and coefficient of viscosity, calculation of σ from η; variation of viscosity with temperature and pressure. Maxwell distribution and i ...
Chemical Reactions - 2012 Book Archive
... example, nitrous oxide, a mild anesthetic, is also used as the propellant in cans of whipped cream, while copper(I) oxide is used as both a red glaze for ceramics and in antifouling bottom paints for boats. In addition to the physical properties of substances, chemists are also interested in their c ...
... example, nitrous oxide, a mild anesthetic, is also used as the propellant in cans of whipped cream, while copper(I) oxide is used as both a red glaze for ceramics and in antifouling bottom paints for boats. In addition to the physical properties of substances, chemists are also interested in their c ...
Chemistry Basics - Mr. Grays Physical Science Class
... Inertia - The inertia of an object represents its ability to resist changes in its motion. This change in motion could be in terms of speed, or in terms of direction. If you are in a car that stops suddenly, you continue to move forward, because of your inertia. When a car makes a sharp turn, you mi ...
... Inertia - The inertia of an object represents its ability to resist changes in its motion. This change in motion could be in terms of speed, or in terms of direction. If you are in a car that stops suddenly, you continue to move forward, because of your inertia. When a car makes a sharp turn, you mi ...
Answers - Pearson
... 6 As NaOH dissolves, the separated Na+ and OH− ions become hydrated, i.e. they are surrounded by H2O molecules. This involves breaking the hydrogen bonds between the H2O molecules in pure water and allows closer packing, which reduces the volume. ...
... 6 As NaOH dissolves, the separated Na+ and OH− ions become hydrated, i.e. they are surrounded by H2O molecules. This involves breaking the hydrogen bonds between the H2O molecules in pure water and allows closer packing, which reduces the volume. ...
Calculations and the Chemical Equation
... Students often have difficulty grasping the meaning of incomplete reactions. This discussion shows the practical economic, as well as scientific ramifications of reactions that have low yields. ...
... Students often have difficulty grasping the meaning of incomplete reactions. This discussion shows the practical economic, as well as scientific ramifications of reactions that have low yields. ...






![mclintock.ch6 [Compatibility Mode]](http://s1.studyres.com/store/data/003971396_1-780a12aa3165c9221aca3ac594a06674-300x300.png)