mark scheme - A-Level Chemistry
... And m/z (1) Multiply m/z by relative abundance for each isotope (1) Allow instead of m/z mass no, Ar or actual value from example Sum these values (1) Divide by the sum of the relative abundances (1) only award this mark if previous 2 given Max 2 if e.g. has only 2 isotopes ...
... And m/z (1) Multiply m/z by relative abundance for each isotope (1) Allow instead of m/z mass no, Ar or actual value from example Sum these values (1) Divide by the sum of the relative abundances (1) only award this mark if previous 2 given Max 2 if e.g. has only 2 isotopes ...
Chemistry
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
Sec. 10.3 - Midland Park School District
... Relate numbers of particles and volumes by using Avogadro’s principle. Recognize the mole relationships shown by a chemical formula. Determine the number of atoms or ions in a mass of a compound. ...
... Relate numbers of particles and volumes by using Avogadro’s principle. Recognize the mole relationships shown by a chemical formula. Determine the number of atoms or ions in a mass of a compound. ...
Chemistry - NIC Karnataka
... SI units to be calculated, value of R in Latm K–1mol–1 to be mentioned. Relation between molar mass and density. Dalton’s law of partial pressures - statement, mathematical form , aqueous tension and pressure of dry gas to be mentioned, relation between partial pressure of a gas and its mole fractio ...
... SI units to be calculated, value of R in Latm K–1mol–1 to be mentioned. Relation between molar mass and density. Dalton’s law of partial pressures - statement, mathematical form , aqueous tension and pressure of dry gas to be mentioned, relation between partial pressure of a gas and its mole fractio ...
organic chemistry - Peoria Public Schools
... Naming of halogenoalkanes In the case of the halogenoalkanes, the name begins with the name of the halogen and not the number of carbon atoms. The name should also indicate the position and the number of halides if there is more than two. ...
... Naming of halogenoalkanes In the case of the halogenoalkanes, the name begins with the name of the halogen and not the number of carbon atoms. The name should also indicate the position and the number of halides if there is more than two. ...
Lecture 11
... • Physics has the same meaning. Except nature ENFORCES the conservation. It’s not optional, or to be fought for. “A force is conservative if the work done by a force on an object moving from one point to another point depends only on the initial and final positions and is independent of the ...
... • Physics has the same meaning. Except nature ENFORCES the conservation. It’s not optional, or to be fought for. “A force is conservative if the work done by a force on an object moving from one point to another point depends only on the initial and final positions and is independent of the ...
Document
... Arrange MgO, CaO, and SrO in order of increasing lattice energy. Strategy Consider the charges on the ions and the distances between them. Apply Coulomb’s law to determine the relative lattice energies. All three compounds contain O2- and all three cations are +2. Recalling that lattice energy incre ...
... Arrange MgO, CaO, and SrO in order of increasing lattice energy. Strategy Consider the charges on the ions and the distances between them. Apply Coulomb’s law to determine the relative lattice energies. All three compounds contain O2- and all three cations are +2. Recalling that lattice energy incre ...
Chapter 6 Thermochemistry
... q = C x DT • the heat capacity of an object depends on its mass 200 g of water requires twice as much heat to raise its temperature by 1°C than 100 g of water ...
... q = C x DT • the heat capacity of an object depends on its mass 200 g of water requires twice as much heat to raise its temperature by 1°C than 100 g of water ...
Honors Chemistry / SAT II
... particles and electrons arranged in concentric shells around the nucleus.” This description most clearly fits the atomic theory proposed by (D) Thomson (A) Bohr (B) Rutherford (E) Avogadro (C) Dalton 2487. The maximum number of electrons possible in the second energy level of an atom is (D) 18 (A) 8 ...
... particles and electrons arranged in concentric shells around the nucleus.” This description most clearly fits the atomic theory proposed by (D) Thomson (A) Bohr (B) Rutherford (E) Avogadro (C) Dalton 2487. The maximum number of electrons possible in the second energy level of an atom is (D) 18 (A) 8 ...
A Sepak Takraw-Based Molecular Model For C60
... Figure 5: Isosurface of ground state electron density for C60 [4] ground state electron density, as calculated by the density functional method. In quantum chemistry this is referred to as a cloud of free electrons. We have so far looked at four current molecular models. So which is correct? In mos ...
... Figure 5: Isosurface of ground state electron density for C60 [4] ground state electron density, as calculated by the density functional method. In quantum chemistry this is referred to as a cloud of free electrons. We have so far looked at four current molecular models. So which is correct? In mos ...
8872 Chemistry H1 syllabus for 2016
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
Chemistry in Context: Chapter 3:The Chemistry of Global Warming
... 0.6 °C from 1880 to 2000; but this may be a short term fluctuation since 120 years are short in comparison to the 4.5 billion history of the Earth. Doubling CO2 levels will increase temperature by 1.0-3.5 °C , smaller than Arrhenius’s prediction of 5-6 in 1896. Absorption of infrared radiation depen ...
... 0.6 °C from 1880 to 2000; but this may be a short term fluctuation since 120 years are short in comparison to the 4.5 billion history of the Earth. Doubling CO2 levels will increase temperature by 1.0-3.5 °C , smaller than Arrhenius’s prediction of 5-6 in 1896. Absorption of infrared radiation depen ...
Chemistry I Exams and Keys Corrected 2016 Season
... 18. Joseph Proust(1754 to 1826) was the chemist to first formally state that: Rejected: because simple memorization. Also, student may not have read about Proust. All full credit. A) When two elements combine with each other to form more than one compound, the weights of one element that combine wi ...
... 18. Joseph Proust(1754 to 1826) was the chemist to first formally state that: Rejected: because simple memorization. Also, student may not have read about Proust. All full credit. A) When two elements combine with each other to form more than one compound, the weights of one element that combine wi ...
Chapters 1-3 Packet
... Come see me if you need help! I will be available before school in 1514. I also have 2nd and 8th periods free. It would be best if you let me know in advance that you are coming since I may have a meeting scheduled. Study Skills: Learning good study skills is one of the most important things that yo ...
... Come see me if you need help! I will be available before school in 1514. I also have 2nd and 8th periods free. It would be best if you let me know in advance that you are coming since I may have a meeting scheduled. Study Skills: Learning good study skills is one of the most important things that yo ...
File
... This is the weight in grams of an ionic (formula) or covalent compound (molecular). To be able to calculate this we need to understand molar mass. First, it is a mass, so it has units of mass, commonly the gram. Second, it concerns the mole (Avogadro's number). Whether you're dealing with elements o ...
... This is the weight in grams of an ionic (formula) or covalent compound (molecular). To be able to calculate this we need to understand molar mass. First, it is a mass, so it has units of mass, commonly the gram. Second, it concerns the mole (Avogadro's number). Whether you're dealing with elements o ...
Unit 10: Structure and Bonding
... In a suitable chemical form, the radioisotope is injected into the body and its 'movement' can be followed. Time is allowed for the radioactive tracer to spread and its progress tracked with a detector outside the body. The patient can be placed next to a 'detection screen' that shows where th ...
... In a suitable chemical form, the radioisotope is injected into the body and its 'movement' can be followed. Time is allowed for the radioactive tracer to spread and its progress tracked with a detector outside the body. The patient can be placed next to a 'detection screen' that shows where th ...
The masses of reactants and products are equal.
... of atoms involved in the reaction. For example, two water molecules (2H2O) contain 2 • 2 = 4 hydrogen atoms and 2 • 1 = 2 oxygen atoms. Remember, coefficients in a chemical equation indicate how many molecules of each type take part in the reaction. Only coefficients can be changed in order to balan ...
... of atoms involved in the reaction. For example, two water molecules (2H2O) contain 2 • 2 = 4 hydrogen atoms and 2 • 1 = 2 oxygen atoms. Remember, coefficients in a chemical equation indicate how many molecules of each type take part in the reaction. Only coefficients can be changed in order to balan ...
Chapter 3 - Stoichiometry
... 69.2% of all copper is copper-63, which has an atomic mass of 62.9296 amu. If all other copper is 65Cu, and the atomic mass of copper is 63.55, what is the atomic mass of copper-65? ...
... 69.2% of all copper is copper-63, which has an atomic mass of 62.9296 amu. If all other copper is 65Cu, and the atomic mass of copper is 63.55, what is the atomic mass of copper-65? ...
Presentation at GE
... Monte Carlo Simulation is used to generate PDF of Heat transfer coefficient ...
... Monte Carlo Simulation is used to generate PDF of Heat transfer coefficient ...
3.091 – Introduction to Solid State Chemistry Lecture Notes No
... by electronic rearrangements must be in a lower energy state than the atoms were prior to interaction, prior to bond formation. Since atoms of each of the elements have different electronic structures, the variety of possible chemical bonds (differing from each other in at least some small way) is c ...
... by electronic rearrangements must be in a lower energy state than the atoms were prior to interaction, prior to bond formation. Since atoms of each of the elements have different electronic structures, the variety of possible chemical bonds (differing from each other in at least some small way) is c ...
i principi di base - Structural Biology
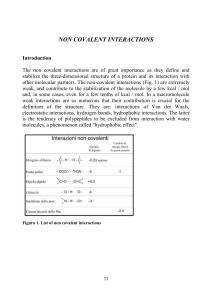
... The hydrogen bond occurs between a donor and an acceptor of hydrogen atoms. When the interaction takes place between charged groups it is often referred to as salt bridge and it has properties typical either of an electrostatic interaction either of an hydrogen bond. The weak bonds between atoms wit ...
... The hydrogen bond occurs between a donor and an acceptor of hydrogen atoms. When the interaction takes place between charged groups it is often referred to as salt bridge and it has properties typical either of an electrostatic interaction either of an hydrogen bond. The weak bonds between atoms wit ...