Chemistry - cloudfront.net
... 32. know that bonding between elements ultimately involves ELECTRONS 33. be able to use a polyatomic sheet to give the formula from a name of an ionic compound, and vice versa 34. understand the definition of valence electrons and how these form bonds between metals (i.e., metallic bonding) and non- ...
... 32. know that bonding between elements ultimately involves ELECTRONS 33. be able to use a polyatomic sheet to give the formula from a name of an ionic compound, and vice versa 34. understand the definition of valence electrons and how these form bonds between metals (i.e., metallic bonding) and non- ...
Chapter 21: Electric Charge and Electric Field
... • Any two conductors separated by an insulator (or a vacuum) form a capacitor • In practice each conductor initially has zero net charge and electrons are transferred from one conductor to the other (charging the conductor) • Then two conductors have charge with equal magnitude and opposite sign, al ...
... • Any two conductors separated by an insulator (or a vacuum) form a capacitor • In practice each conductor initially has zero net charge and electrons are transferred from one conductor to the other (charging the conductor) • Then two conductors have charge with equal magnitude and opposite sign, al ...
PHYSICAL SETTING CHEMISTRY
... questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. All answers in your answer booklet should be written in pen, except for graphs and drawings, whic ...
... questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. All answers in your answer booklet should be written in pen, except for graphs and drawings, whic ...
1 Course Code– CH1141 Semester – I Credit
... Module II - Chemical bonding – Energetic of bond formation – Types of Chemical bonds – Energetics of ionic bond formation – Lattice energy – Born Haber Cycle – partial covalent nature of ionic bond – Fajan’s rules , polarity of covalent bond its relation with electronegativity – electro negativity s ...
... Module II - Chemical bonding – Energetic of bond formation – Types of Chemical bonds – Energetics of ionic bond formation – Lattice energy – Born Haber Cycle – partial covalent nature of ionic bond – Fajan’s rules , polarity of covalent bond its relation with electronegativity – electro negativity s ...
CHAPTER 10 CHEMICAL BONDING II: MOLECULAR GEOMETRY
... The molecules shown in (b) and (d) are nonpolar. Due to the high symmetry of the molecules and the equal magnitude of the bond moments, the bond moments in each molecule cancel one another. The resultant dipole moment will be zero. For the molecules shown in (a) and (c), the bond moments do not canc ...
... The molecules shown in (b) and (d) are nonpolar. Due to the high symmetry of the molecules and the equal magnitude of the bond moments, the bond moments in each molecule cancel one another. The resultant dipole moment will be zero. For the molecules shown in (a) and (c), the bond moments do not canc ...
Unit3_Stoichiometry_vs2
... (Note though that they will both have the same value for average kinetic energy.) 7 From the kinetic molecular theory we would expect a solid to be more dense than its liquid, and therefore that ice would sink in water. ...
... (Note though that they will both have the same value for average kinetic energy.) 7 From the kinetic molecular theory we would expect a solid to be more dense than its liquid, and therefore that ice would sink in water. ...
Chapter 3 Stoichiometry: Calculations with Chemical
... Explanation: This is based on reading the formula and correctly extracting information from it. The formula C2H6SO indicates that every mole of this compound has 2 moles of carbon atoms in it. Thus 4 moles of the compound would have 4 x 2 = 8 moles of C atoms. 13. There are ______ sulfur atoms in 25 ...
... Explanation: This is based on reading the formula and correctly extracting information from it. The formula C2H6SO indicates that every mole of this compound has 2 moles of carbon atoms in it. Thus 4 moles of the compound would have 4 x 2 = 8 moles of C atoms. 13. There are ______ sulfur atoms in 25 ...
James Moir as Inorganic Chemist
... The structures of Ag(tu)2Cl and Au(tu)2Br have also been determined and shown to be similarly complex compounds.13,14 Moir succeeded in isolating two new compounds of gold and thiourea: one which he obtained from gold in a solution of thiourea with sulfuric acid and hydrogen peroxide, which he analy ...
... The structures of Ag(tu)2Cl and Au(tu)2Br have also been determined and shown to be similarly complex compounds.13,14 Moir succeeded in isolating two new compounds of gold and thiourea: one which he obtained from gold in a solution of thiourea with sulfuric acid and hydrogen peroxide, which he analy ...
Click here for presentation
... produced using the excited states of positronium, Ps(2p) and Ps(2s) [7] . This would require defining the H and Ps states in such a way that: • The n=2 energy states are as expected • The n=2 cross-sections produce smooth ...
... produced using the excited states of positronium, Ps(2p) and Ps(2s) [7] . This would require defining the H and Ps states in such a way that: • The n=2 energy states are as expected • The n=2 cross-sections produce smooth ...
Chapter 6 - Sites @ Suffolk University
... chemical equation, in which a great deal of useful information is contained in a simple form. In our chemical reaction of hydrogen and oxygen to form water, we say that the hydrogen and oxygen are the reactants, and water is the reaction product. The reactants appear to the left of our chemical equa ...
... chemical equation, in which a great deal of useful information is contained in a simple form. In our chemical reaction of hydrogen and oxygen to form water, we say that the hydrogen and oxygen are the reactants, and water is the reaction product. The reactants appear to the left of our chemical equa ...
Chapter 2: Mass Relations in Formulas, Chemical Reactions, and
... equation is the number of atoms, ions, formula units or molecules associated with each substance. The number in front of each substance is called the stoichiometric coefficients or more simply the coefficient. The bulk of this information is often referred to as the stoichiometry of the chemical rea ...
... equation is the number of atoms, ions, formula units or molecules associated with each substance. The number in front of each substance is called the stoichiometric coefficients or more simply the coefficient. The bulk of this information is often referred to as the stoichiometry of the chemical rea ...
Chapter 3 Notes
... Chapter 3 College Chemistry Chemical Equations and Stoichiometry Stoichiometry: the study of quantities of substances used and produced in a chemical equation. Stoichiometry is based on… chemical equations represent chemical reactions Law of Conservation of Matter: Matter (mass) cannot be create ...
... Chapter 3 College Chemistry Chemical Equations and Stoichiometry Stoichiometry: the study of quantities of substances used and produced in a chemical equation. Stoichiometry is based on… chemical equations represent chemical reactions Law of Conservation of Matter: Matter (mass) cannot be create ...
5. Stoichiometry - Sakshi Education
... c) In acid medium include enough number of water molecules where there is a deficiency of oxygen and include enough number of H + ions on the side where there is a deficiency of Hydrogen. d) In alkaline medium include enough number of OH⎯ ions on the side where there is a deficiency of oxygen and en ...
... c) In acid medium include enough number of water molecules where there is a deficiency of oxygen and include enough number of H + ions on the side where there is a deficiency of Hydrogen. d) In alkaline medium include enough number of OH⎯ ions on the side where there is a deficiency of oxygen and en ...
Noninteracting Particle Systems - Particle Solids Interactions group
... the interaction between the spins in an Ising model can be neglected because the mean energy exchanged with the heat bath is much larger than the potential energy of interaction. Another reason for studying systems of noninteracting particles is that there are many cases for which the equilibrium pr ...
... the interaction between the spins in an Ising model can be neglected because the mean energy exchanged with the heat bath is much larger than the potential energy of interaction. Another reason for studying systems of noninteracting particles is that there are many cases for which the equilibrium pr ...
Lecture Notes for Section 15.1 (Impulse & Momentum)
... PRINCIPLE OF LINEAR IMPULSE AND MOMENTUM (continued) The next method we will consider for solving particle kinetics problems is obtained by integrating the equation of motion with respect to time. The result is referred to as the principle of impulse and momentum. It can be applied to problems invo ...
... PRINCIPLE OF LINEAR IMPULSE AND MOMENTUM (continued) The next method we will consider for solving particle kinetics problems is obtained by integrating the equation of motion with respect to time. The result is referred to as the principle of impulse and momentum. It can be applied to problems invo ...
Chemistry - Beachwood City Schools
... between the levels. The greater the energy difference, the shorter the wavelength of light, the more violet the color. 3. The electron configurations of all Group 1 metals end with a single s electron. When these metals lose this s electron, they acquire noble gas electron configurations which end i ...
... between the levels. The greater the energy difference, the shorter the wavelength of light, the more violet the color. 3. The electron configurations of all Group 1 metals end with a single s electron. When these metals lose this s electron, they acquire noble gas electron configurations which end i ...
Chemistry - Pearson School
... Key Idea 3: The grouping of magnitudes of size, time, frequency, and pressures or other units of measurement into a series of relative order provides a useful way to deal with the immense range and the changes in scale that affect the behavior and design of systems. 3.1 Describe the effects of chang ...
... Key Idea 3: The grouping of magnitudes of size, time, frequency, and pressures or other units of measurement into a series of relative order provides a useful way to deal with the immense range and the changes in scale that affect the behavior and design of systems. 3.1 Describe the effects of chang ...
AP Chemistry: Bonding Multiple Choice
... (A) are made up of atoms that are intrinsically hard because of their electronic structures (B) consist of positive and negative ions that are strongly attracted to each other (C) are giant molecules in which each atom forms strong covalent bonds with all of its neighboring atoms (D) are formed unde ...
... (A) are made up of atoms that are intrinsically hard because of their electronic structures (B) consist of positive and negative ions that are strongly attracted to each other (C) are giant molecules in which each atom forms strong covalent bonds with all of its neighboring atoms (D) are formed unde ...
Molecular geometry
... that is slightly altered or perturbed by some additional force or interaction (such as the interaction between the two atoms). Variational method (used in molecular orbital theory): The energy of a trial function (educated function) within the Schrodinger equation is minimized. ...
... that is slightly altered or perturbed by some additional force or interaction (such as the interaction between the two atoms). Variational method (used in molecular orbital theory): The energy of a trial function (educated function) within the Schrodinger equation is minimized. ...
File
... A crystalline solid with a high melting point which conducts electricity only when molten or dissolved in water is: a. a molecular compound c. a metal b. an ionic compound d. a network covalent solid ...
... A crystalline solid with a high melting point which conducts electricity only when molten or dissolved in water is: a. a molecular compound c. a metal b. an ionic compound d. a network covalent solid ...
Chemistry
... 2.1 Identify scientific problems, observe phenomena and pose scientific questions/hypotheses 2.2 Plan and conduct investigations by selecting the appropriate experimental procedures, apparatus and materials, with due regard for accuracy, precision and safety 2.3 Obtain, organise and represent data i ...
... 2.1 Identify scientific problems, observe phenomena and pose scientific questions/hypotheses 2.2 Plan and conduct investigations by selecting the appropriate experimental procedures, apparatus and materials, with due regard for accuracy, precision and safety 2.3 Obtain, organise and represent data i ...
Lecture 23
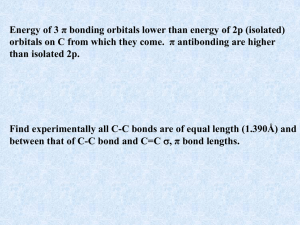
... between the bonding and anti-bonding orbitals is much smaller than for the insulator carbon (diamond). ...
... between the bonding and anti-bonding orbitals is much smaller than for the insulator carbon (diamond). ...