Chemical bonding
... release of electrons from the metal and formation of cation is Na → Na+ + e – 3) Ans: NaCl is more stable than KCl. Lattice energy of NaCl (788KJ/mol) greater than lattice energy of KCl (718KJ/mol) because smaller ionic radius of Na+ (95pm) when compared to K+ (133pm). 4)Ans: According to the concep ...
... release of electrons from the metal and formation of cation is Na → Na+ + e – 3) Ans: NaCl is more stable than KCl. Lattice energy of NaCl (788KJ/mol) greater than lattice energy of KCl (718KJ/mol) because smaller ionic radius of Na+ (95pm) when compared to K+ (133pm). 4)Ans: According to the concep ...
10.2The Mole-Mass Relationship
... Converting from moles of a compound to grams Example: I need 3.00 mol NaCl for an experiment. How many grams is that? Step 1: Find the molar mass Molar mass = 22.09g/mol + 35.45g/mol = 57.54 g/mol Step 2: Use the molar mass like a conversion factor. ...
... Converting from moles of a compound to grams Example: I need 3.00 mol NaCl for an experiment. How many grams is that? Step 1: Find the molar mass Molar mass = 22.09g/mol + 35.45g/mol = 57.54 g/mol Step 2: Use the molar mass like a conversion factor. ...
week09.1.suspensions
... o = density of continuous phase o = viscosity of continuous phase (Pa s) ...
... o = density of continuous phase o = viscosity of continuous phase (Pa s) ...
Summer Assignment: Some Review / Basic Prep
... (scales with mass) making the density an intensive property, or independent of the system's size. Thus, (and here’s the interesting point….) when two extensive properties are divided by each other the result is an intensive property e.g ... Mass (extensive) = Density (intensive) I think that is sort ...
... (scales with mass) making the density an intensive property, or independent of the system's size. Thus, (and here’s the interesting point….) when two extensive properties are divided by each other the result is an intensive property e.g ... Mass (extensive) = Density (intensive) I think that is sort ...
Topic 4
... In 1954 Linus Pauling was awarded the Chemistry Nobel Prize for his work on the nature of the chemical bond. Covalent bonds are one example of intramolecular bonding. Explain the formation of the following. (i) ...
... In 1954 Linus Pauling was awarded the Chemistry Nobel Prize for his work on the nature of the chemical bond. Covalent bonds are one example of intramolecular bonding. Explain the formation of the following. (i) ...
Formulae Boardwork
... This concept is useful to chemical industry, because it takes into account the atoms that end up in unwanted waste products as well as the yield of the reaction. This means a process that produces several worthless by-products could have a high yield but a low atom economy. Reactions with a high ato ...
... This concept is useful to chemical industry, because it takes into account the atoms that end up in unwanted waste products as well as the yield of the reaction. This means a process that produces several worthless by-products could have a high yield but a low atom economy. Reactions with a high ato ...
Changes in Matter: Physical and Chemical Changes
... You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole problem. 4. Write numbers in front of each of the boxes until the inventory for each element is the same both before and after the reaction. Whenever you change a numbe ...
... You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole problem. 4. Write numbers in front of each of the boxes until the inventory for each element is the same both before and after the reaction. Whenever you change a numbe ...
9647 H2 Chemistry
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus Content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus Content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
Note Sheets and Sample Problems
... o e is charge on electron in Coulombs, (C) and m is its mass. o Thomson discovered that he could repeat this deflection and calculation using electrodes of different metals ∴ all metals contained electrons and ALL ATOMS contained electrons o Furthermore, all atoms were neutral ∴ there must be some ( ...
... o e is charge on electron in Coulombs, (C) and m is its mass. o Thomson discovered that he could repeat this deflection and calculation using electrodes of different metals ∴ all metals contained electrons and ALL ATOMS contained electrons o Furthermore, all atoms were neutral ∴ there must be some ( ...
Brochure BITSAT-2011
... to score higher. However, candidates should keep in mind the fact that there is negative marking for wrong answers and any attempt to answer the questions by pure guessing of the answers is not likely to have any advantage, but may result in a reduction in the total score. The questions will be sele ...
... to score higher. However, candidates should keep in mind the fact that there is negative marking for wrong answers and any attempt to answer the questions by pure guessing of the answers is not likely to have any advantage, but may result in a reduction in the total score. The questions will be sele ...
Chemical Reaction
... involves the rearrangement of atoms. produces one or more new substances. can be observed by the appearance of new physical properties. A chemical reaction forms new products with different properties. An antacid (NaHCO3) tablet in water forms bubbles of carbon dioxide (CO2). © 2013 Pearson Ed ...
... involves the rearrangement of atoms. produces one or more new substances. can be observed by the appearance of new physical properties. A chemical reaction forms new products with different properties. An antacid (NaHCO3) tablet in water forms bubbles of carbon dioxide (CO2). © 2013 Pearson Ed ...
File - Science with Mr. Louie
... o As a rule, when performing a series of calculations, wait until the very end to round off to the proper number of significant figures instead of rounding off each intermediate result. If you are changing from addition /subtraction to multiplication/division or vice versa, note the number of sig fi ...
... o As a rule, when performing a series of calculations, wait until the very end to round off to the proper number of significant figures instead of rounding off each intermediate result. If you are changing from addition /subtraction to multiplication/division or vice versa, note the number of sig fi ...
Stoichiometry w RICE
... Sulfuric acid is formed when sulfur dioxide reacts with oxygen and water. Write the balanced chemical equation for the reaction. If 12.5 mol SO2 reacts, how many moles H2SO4 can be produced? How many mole O2 is needed? ...
... Sulfuric acid is formed when sulfur dioxide reacts with oxygen and water. Write the balanced chemical equation for the reaction. If 12.5 mol SO2 reacts, how many moles H2SO4 can be produced? How many mole O2 is needed? ...
IPC: Essential Learning Outcomes By the IPC District Team
... • Name and describe the six simple machines. • Explain and calculate the I.M.A and A.M.A of simple machines. • Explain and calculate the efficiency of machines. • Define and calculate potential and kinetic energy. • Identify and describe transformations of energy. X. Temperature and Heat TLW: • Expl ...
... • Name and describe the six simple machines. • Explain and calculate the I.M.A and A.M.A of simple machines. • Explain and calculate the efficiency of machines. • Define and calculate potential and kinetic energy. • Identify and describe transformations of energy. X. Temperature and Heat TLW: • Expl ...
CHEMISTRY – Summer Assignment Solutions 2013
... Naming – always name the ions not the formulas (cation then anion). Name tells the type of ions involved not how many of each ion cations: name the element; if more than one oxidation state is possible (d-block) follow with the charge in Roman numerals in parentheses anions: if monatomic then use ...
... Naming – always name the ions not the formulas (cation then anion). Name tells the type of ions involved not how many of each ion cations: name the element; if more than one oxidation state is possible (d-block) follow with the charge in Roman numerals in parentheses anions: if monatomic then use ...
NOBLE-GAS CHEMISTRY
... Cr(0) should of course be much less prone to auxiliary bonding than Be(II) (even if coordinatively unsaturated) but five CO p-acceptors are capable of withdrawing a large share of the electron density from Cr(0). The bonding of xenon to M(0) in XeM(CO)5 is weaker than the respective bonding of H2 or ...
... Cr(0) should of course be much less prone to auxiliary bonding than Be(II) (even if coordinatively unsaturated) but five CO p-acceptors are capable of withdrawing a large share of the electron density from Cr(0). The bonding of xenon to M(0) in XeM(CO)5 is weaker than the respective bonding of H2 or ...
Congratulations! You have signed up for AP Chemistry for this year
... 400 B.C.—Greeks—proposed all matter was make up of 4 “elements” : fire, earth, water and air Democritus—first to use the term atomos to describe the ultimate, smallest particles of matter Next 2,000 years—alchemy—a pseudoscience where people thought they could turn metals into gold. Some good chemis ...
... 400 B.C.—Greeks—proposed all matter was make up of 4 “elements” : fire, earth, water and air Democritus—first to use the term atomos to describe the ultimate, smallest particles of matter Next 2,000 years—alchemy—a pseudoscience where people thought they could turn metals into gold. Some good chemis ...
Calculations with Chemical Formulas and Equations Chapter 3
... The limiting reactant (or limiting reagent) is the reactant that is entirely consumed when the reaction goes to completion. The limiting reagent ultimately determines how much product can be obtained. For example, bicycles require one frame and two wheels. If you have 20 wheels but only 5 frames, it ...
... The limiting reactant (or limiting reagent) is the reactant that is entirely consumed when the reaction goes to completion. The limiting reagent ultimately determines how much product can be obtained. For example, bicycles require one frame and two wheels. If you have 20 wheels but only 5 frames, it ...
1. Which idea of John Dalton is no longer considered part of the
... The pressure is reduced by 1 . ...
... The pressure is reduced by 1 . ...
Wk2_Monday
... formed? The reactant which is first used up determines the amount of product formed. This reactants is called the LIMITING REACTANT. The other reactants are in EXCESS when the reaction stops. If additional amounts of the limiting reactant is added the reaction starts again. ...
... formed? The reactant which is first used up determines the amount of product formed. This reactants is called the LIMITING REACTANT. The other reactants are in EXCESS when the reaction stops. If additional amounts of the limiting reactant is added the reaction starts again. ...
Chemical Measurements
... • The number of molecules in 1mole of a molecular compound is 6.02 x 1023 (same as with atoms in a mole) • 1 mol of water (H2O) contains 1 mol of water molecules but 2 mol of hydrogen atoms and 1 mol oxygen. • How many moles of Ca2+ and F- are in 1 mole of calcium fluoride (CaF2)? o ...
... • The number of molecules in 1mole of a molecular compound is 6.02 x 1023 (same as with atoms in a mole) • 1 mol of water (H2O) contains 1 mol of water molecules but 2 mol of hydrogen atoms and 1 mol oxygen. • How many moles of Ca2+ and F- are in 1 mole of calcium fluoride (CaF2)? o ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • Remember, the number of atoms in a molecular formula is a multiple of the number of atoms in an empirical formula. • If we find the empirical formula and know a molar mass (molecular weight) for the compound, we can find the molecular formula. Stoichiometry © 2015 Pearson Education ...
... • Remember, the number of atoms in a molecular formula is a multiple of the number of atoms in an empirical formula. • If we find the empirical formula and know a molar mass (molecular weight) for the compound, we can find the molecular formula. Stoichiometry © 2015 Pearson Education ...
The mole and calculations
... Molar Mass: the mass of a substance per 1 mole of its entities (atoms, molecules, ions, or formula units). Units for molar mass are grams/mole. Once again, the periodic table is used to calculate the molar mass (previously we called this molecular mass - it's the same thing!!) 1.) to find the mo ...
... Molar Mass: the mass of a substance per 1 mole of its entities (atoms, molecules, ions, or formula units). Units for molar mass are grams/mole. Once again, the periodic table is used to calculate the molar mass (previously we called this molecular mass - it's the same thing!!) 1.) to find the mo ...
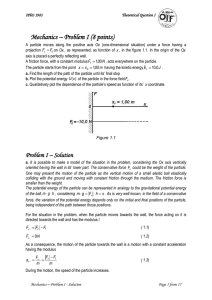
Solution
... a. It is possible to make a model of the situation in the problem, considering the Ox axis vertically oriented having the wall in its’ lower part. The conservative force Fx could be the weight of the particle. One may present the motion of the particle as the vertical motion of a small elastic ball ...
... a. It is possible to make a model of the situation in the problem, considering the Ox axis vertically oriented having the wall in its’ lower part. The conservative force Fx could be the weight of the particle. One may present the motion of the particle as the vertical motion of a small elastic ball ...
Covalent Bonding and Nomenclature
... to attract electrons when chemically combining with another element. The higher the electronegativity value, the stronger the attraction the atom has for another atom’s electrons. ...
... to attract electrons when chemically combining with another element. The higher the electronegativity value, the stronger the attraction the atom has for another atom’s electrons. ...