MATHEMATICAL HISTORY OF WAVE AND MATRIX QUANTUM
... The knowledge of atomic structure was reached through the discovery of the electron, due to J. J. Thomson, and through the discovery of the atomic nucleus, which we owe to E. Rutherford, by studying radioactive substances and working with α- and β-particles. Towards 1910, experimental evidence exist ...
... The knowledge of atomic structure was reached through the discovery of the electron, due to J. J. Thomson, and through the discovery of the atomic nucleus, which we owe to E. Rutherford, by studying radioactive substances and working with α- and β-particles. Towards 1910, experimental evidence exist ...
2.3 Lecture 7: Binary collisions
... which is not very useful unless ˆf is specified. Of course, finding or modˆt coll eling the collision term is the biggest challenge in the kinetic theory. In the simplest model one only takes into account binary collisions and assumes that the colliding particles are uncorrelated (i.e. molecular cha ...
... which is not very useful unless ˆf is specified. Of course, finding or modˆt coll eling the collision term is the biggest challenge in the kinetic theory. In the simplest model one only takes into account binary collisions and assumes that the colliding particles are uncorrelated (i.e. molecular cha ...
Electron Scattering Intensities and Patterson Functions of Skyrmions
... Variants with small oscillations and a central depression are also used, particularly for larger nuclei [7]. However, these variants require more parameters to fit the data closely and do not have a theoretical grounding which explains their values. We wish to see whether the Skyrme model can provi ...
... Variants with small oscillations and a central depression are also used, particularly for larger nuclei [7]. However, these variants require more parameters to fit the data closely and do not have a theoretical grounding which explains their values. We wish to see whether the Skyrme model can provi ...
Document
... SORT The problem gives the mass of carbon dioxide and asks you to find the mass of glucose that can be produced. ...
... SORT The problem gives the mass of carbon dioxide and asks you to find the mass of glucose that can be produced. ...
Sources of Parallelism and Locality in Simulation
... Sharks and Fish • S&F 1. Fish alone move continuously subject to an external current and Newton's laws. • S&F 2. Fish alone move continuously subject to gravitational attraction and Newton's laws. • S&F 3. Fish alone play the "Game of Life" on a square grid. • S&F 4. Fish alone move randomly on a s ...
... Sharks and Fish • S&F 1. Fish alone move continuously subject to an external current and Newton's laws. • S&F 2. Fish alone move continuously subject to gravitational attraction and Newton's laws. • S&F 3. Fish alone play the "Game of Life" on a square grid. • S&F 4. Fish alone move randomly on a s ...
Chapter 11 PPT
... the plane formed by the position vector and the force vector Fr The torque is the vector (or cross) product of the position vector and the force vector ...
... the plane formed by the position vector and the force vector Fr The torque is the vector (or cross) product of the position vector and the force vector ...
Measurement and assignment of the size-dependent
... band ~G7!. ~The subscripts, 3/2 or 1/2, describe the total unit cell angular momentum, J5l1s.! Away from k50 the J53/2 band splits further into the heavy-hole ~J m 563/2! and light-hole ~J m 561/2! bands, both doubly degenerate.33 More recent quantum dot theoretical work14,32,34–37 considers the val ...
... band ~G7!. ~The subscripts, 3/2 or 1/2, describe the total unit cell angular momentum, J5l1s.! Away from k50 the J53/2 band splits further into the heavy-hole ~J m 563/2! and light-hole ~J m 561/2! bands, both doubly degenerate.33 More recent quantum dot theoretical work14,32,34–37 considers the val ...
ABSTRACT PHOTON PAIR PRODUCTION FROM A HOT ATOMIC ENSEMBLE IN THE DIAMOND CONFIGURATION
... Quantum mechanically correlated photon pairs, as well as single photon pulses have numerous uses in the field of quantum information science. These include quantum encryption/communicaiton [31] and linear optical quantum computation [35]. The motivation for the work presented here in this thesis is ...
... Quantum mechanically correlated photon pairs, as well as single photon pulses have numerous uses in the field of quantum information science. These include quantum encryption/communicaiton [31] and linear optical quantum computation [35]. The motivation for the work presented here in this thesis is ...
Microscopic theory for quantum mirages in quantum corrals D. Porras, J. Ferna´ndez-Rossier,
... construction of quantum structures of arbitrary shape. Additionally, the differential conductance, G(V)⬅dI/dV, is proportional to the local density of states 共LDOS兲 of the surface spot below the tip.2 Hence, STM can be used to modify and to measure the LDOS. A STM was used by Crommie et al. to build ...
... construction of quantum structures of arbitrary shape. Additionally, the differential conductance, G(V)⬅dI/dV, is proportional to the local density of states 共LDOS兲 of the surface spot below the tip.2 Hence, STM can be used to modify and to measure the LDOS. A STM was used by Crommie et al. to build ...
The Thomas-Fermi model: momentum expectation values
... Within the Thomas-Fermi model including the exchange interaction and contributions of strongly bound electrons, analytical expressions are obtained for all momentum expectation values pb> and for some of the expectation values of powers of the electron density pm> for an atom with an arbit ...
... Within the Thomas-Fermi model including the exchange interaction and contributions of strongly bound electrons, analytical expressions are obtained for all momentum expectation values pb> and for some of the expectation values of powers of the electron density pm> for an atom with an arbit ...
Classical Mechanics
... the sense, that a motion of a particle, a body, or a collection of them are studied without regard to why it happens, whereas kinetics deals with the motion in relation to its causes, i.e. forces and torques. For example, a particle shot up at an angle under a constant vertical downward acceleration ...
... the sense, that a motion of a particle, a body, or a collection of them are studied without regard to why it happens, whereas kinetics deals with the motion in relation to its causes, i.e. forces and torques. For example, a particle shot up at an angle under a constant vertical downward acceleration ...
Physics 2009
... b. Students know that when forces are balanced, no acceleration occurs; thus an object continues to move at a constant speed or stays at rest (Newton’s first law). c. Students know how to apply the law FÊ =Ê ma to solve one-dimensional motion problems that involve constant forces (Newton’s second la ...
... b. Students know that when forces are balanced, no acceleration occurs; thus an object continues to move at a constant speed or stays at rest (Newton’s first law). c. Students know how to apply the law FÊ =Ê ma to solve one-dimensional motion problems that involve constant forces (Newton’s second la ...
Multi-particle simulation code for IBS - Indico
... Conventional IBS theories in Accelerator Physics (Bjorken-Mtingwa, Piwinski) derive Tk by the formula: ...
... Conventional IBS theories in Accelerator Physics (Bjorken-Mtingwa, Piwinski) derive Tk by the formula: ...
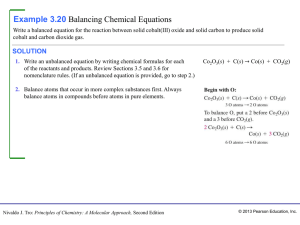
Section 2 Types of Chemical Reactions
... • Begin by counting carbon atoms. • Carbon is already balanced in the equation. • Two additional hydrogen atoms are needed on the right side of the equation. • Now increase the oxygen atoms by placing the coefficient 2 in front of the molecular formula for oxygen. The correct chemical equation, or b ...
... • Begin by counting carbon atoms. • Carbon is already balanced in the equation. • Two additional hydrogen atoms are needed on the right side of the equation. • Now increase the oxygen atoms by placing the coefficient 2 in front of the molecular formula for oxygen. The correct chemical equation, or b ...
Impulse and Momentum Review
... 7.2 The Principle of Conservation of Linear Momentum We’ve seen that if you want to change the momentum of an object or a system of objects, Newton’s second law says that you have to apply an unbalanced force. This implies that if there are no unbalanced forces acting on a system, the total momentum ...
... 7.2 The Principle of Conservation of Linear Momentum We’ve seen that if you want to change the momentum of an object or a system of objects, Newton’s second law says that you have to apply an unbalanced force. This implies that if there are no unbalanced forces acting on a system, the total momentum ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.