Exam 2 Review - Iowa State University
... 4. The sum of oxidation numbers of all atoms in a compound equal the charge of that compound 1. Identify the oxidation number of each individual atom in the following equation. a. Which atom is being oxidized? Reduced? Zn (s) + H2SO4 (aq) ZnSO4 (aq) + H2 (g) ...
... 4. The sum of oxidation numbers of all atoms in a compound equal the charge of that compound 1. Identify the oxidation number of each individual atom in the following equation. a. Which atom is being oxidized? Reduced? Zn (s) + H2SO4 (aq) ZnSO4 (aq) + H2 (g) ...
Day 5 Intro-to-Chem
... S Was your hypothesis supported or not (try NOT to think of it as success vs failure)? S How could you improve your experiment? S How could you expand upon your original ...
... S Was your hypothesis supported or not (try NOT to think of it as success vs failure)? S How could you improve your experiment? S How could you expand upon your original ...
Modern Physics
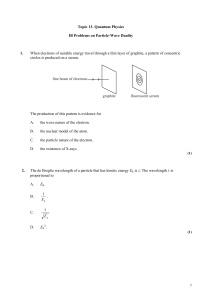
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
Modern Physics
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
Posttest for Uncertainty Principle Part 1
... 1. Ignore normalization issues pertaining to the wave function. At time t=0, the wave packet of a quantum mechanical particle is highly peaked and can be effectively described by a delta function (x) . Is the momentum of this particle well-defined at t=0? Is the position of the particle well-defin ...
... 1. Ignore normalization issues pertaining to the wave function. At time t=0, the wave packet of a quantum mechanical particle is highly peaked and can be effectively described by a delta function (x) . Is the momentum of this particle well-defined at t=0? Is the position of the particle well-defin ...
che-20028 QC lecture 2 - Rob Jackson`s Website
... – As temperature is lowered to absolute zero, helium remains a liquid, rather than freezing to a solid, because of its zero-point energy. – Very high pressures are needed to freeze it. ...
... – As temperature is lowered to absolute zero, helium remains a liquid, rather than freezing to a solid, because of its zero-point energy. – Very high pressures are needed to freeze it. ...
Exam #: Printed Name: Signature: PHYSICS DEPARTMENT
... (a) Obtain the Lagrangian for this constrained motion. For definiteness, start from spherical polar coordinates with the z-axis parallel to the gravitational field. (b) Derive the Hamiltonian function H using the same coordinates as in (a). (c) Calculate the total energy E of the particle. Is E = H? ...
... (a) Obtain the Lagrangian for this constrained motion. For definiteness, start from spherical polar coordinates with the z-axis parallel to the gravitational field. (b) Derive the Hamiltonian function H using the same coordinates as in (a). (c) Calculate the total energy E of the particle. Is E = H? ...
Specification
... The term, ‘number of moles’ is to be avoided. The term, ‘amount of substance in moles’ is preferred. In the same manner, the size of an object can be described in terms of its ‘length in metres’, rather than its ‘number of metres’. Graph Axes and Table Headings Labelled as: quantity / unit, e.g. c / ...
... The term, ‘number of moles’ is to be avoided. The term, ‘amount of substance in moles’ is preferred. In the same manner, the size of an object can be described in terms of its ‘length in metres’, rather than its ‘number of metres’. Graph Axes and Table Headings Labelled as: quantity / unit, e.g. c / ...
File
... b) Nucleus: the dense centre region of an atom. It contains the protons and neutrons (if there are any). c) Proton: a sub-atomic particle that is found it nucleus of an atom. Protons have a charge of 1+ and a mass of 1 amu. d) Neutron: a sub-atomic particle that is found in the nucleus of an atom. N ...
... b) Nucleus: the dense centre region of an atom. It contains the protons and neutrons (if there are any). c) Proton: a sub-atomic particle that is found it nucleus of an atom. Protons have a charge of 1+ and a mass of 1 amu. d) Neutron: a sub-atomic particle that is found in the nucleus of an atom. N ...
IO-IY
... shell? Refer to Figure 30.17 for the order in which the subshells fill. Section 30.7 X-Rays 33. ssm Molybdenum has an atomic number of 2 = 42. Using the Bohr model, estimate the wavelength of the Kcx X-ray. 34. Interactive LearningWare 30.2 at www.wiley.comlcollege/cutnell reviews the concepts that ...
... shell? Refer to Figure 30.17 for the order in which the subshells fill. Section 30.7 X-Rays 33. ssm Molybdenum has an atomic number of 2 = 42. Using the Bohr model, estimate the wavelength of the Kcx X-ray. 34. Interactive LearningWare 30.2 at www.wiley.comlcollege/cutnell reviews the concepts that ...
2 is
... En= -13.6 Z2/n2 • Photon absorbed when electron j jumps from f low l energy to t high hi h energy. Photon emitted when electron l t jumps j from f high hi h energy to t low energy orbit: E2 – E1 = h f = h c / λ Physics 102: Lecture 25, Slide 3 ...
... En= -13.6 Z2/n2 • Photon absorbed when electron j jumps from f low l energy to t high hi h energy. Photon emitted when electron l t jumps j from f high hi h energy to t low energy orbit: E2 – E1 = h f = h c / λ Physics 102: Lecture 25, Slide 3 ...
General, Organic, and Biological Chemistry
... 46) Isotopes are atoms of the same element that have A) different atomic numbers. B) the same atomic numbers but different numbers of protons. C) the same atomic numbers but different numbers of electrons. D) the same atomic number but different numbers of neutrons. E) the same atomic mass but diff ...
... 46) Isotopes are atoms of the same element that have A) different atomic numbers. B) the same atomic numbers but different numbers of protons. C) the same atomic numbers but different numbers of electrons. D) the same atomic number but different numbers of neutrons. E) the same atomic mass but diff ...
LESSON 9
... As we look out at the universe, we are looking back in time because light had to leave distant objects a long time ago, to reach us at the present time. This means that the events we observe lie on what is called our past light cone. The point of the cone is at our position, at the present time. As ...
... As we look out at the universe, we are looking back in time because light had to leave distant objects a long time ago, to reach us at the present time. This means that the events we observe lie on what is called our past light cone. The point of the cone is at our position, at the present time. As ...
Re-typed from The Ultimate Chemical Equations Handbook by
... Re-typed from The Ultimate Chemical Equations Handbook by Hague and Smith Ternary Nomenclature: Acids and salts Containing Halogens and/or Oxygen 1. The halogens, with their variable oxidation numbers, allow for a great variety of compounds. 2. A good way to learn ternary nomenclature is to start ...
... Re-typed from The Ultimate Chemical Equations Handbook by Hague and Smith Ternary Nomenclature: Acids and salts Containing Halogens and/or Oxygen 1. The halogens, with their variable oxidation numbers, allow for a great variety of compounds. 2. A good way to learn ternary nomenclature is to start ...
mass spectrometry
... What should you be able to do? Recall the four basic stages in obtaining a mass spectrum Understand what happens during each of the above four stages Understand why particles need to be in the form of ions ...
... What should you be able to do? Recall the four basic stages in obtaining a mass spectrum Understand what happens during each of the above four stages Understand why particles need to be in the form of ions ...
Quantum theory or radiation
... We can use this idea to calculate the number of photons (a word used to describe a quantum of visible radiation) emitted by a 100 W yellow light per second. Energy emitted by the light bulb every second = 100 J Energy of each quantum = 3.31x10-19 J Therefore number emitted per second = 100/3.31x10-1 ...
... We can use this idea to calculate the number of photons (a word used to describe a quantum of visible radiation) emitted by a 100 W yellow light per second. Energy emitted by the light bulb every second = 100 J Energy of each quantum = 3.31x10-19 J Therefore number emitted per second = 100/3.31x10-1 ...
Physics 422 - Spring 2013 - Midterm Exam, March 6
... The cylinder has a total mass, M , and a cross sectional area A and the water, which has density ρ, provides an upward buoyant force on the cylinder. You can assume that some part of the cylinder always remains under water and that the cylinder is never completely submerged. Suppose that the distanc ...
... The cylinder has a total mass, M , and a cross sectional area A and the water, which has density ρ, provides an upward buoyant force on the cylinder. You can assume that some part of the cylinder always remains under water and that the cylinder is never completely submerged. Suppose that the distanc ...
Course summary for Unit 4 "Interactions of Light and
... Interpret electron diffraction patterns as evidence for the wave-like nature of matter expressed as the de Broglie wavelength = h/p; Momentum of A Photon In the Photon model, photons have energy like a particle, can a photon have momentum? Maxwell had said that an electromagnetic wave which was ca ...
... Interpret electron diffraction patterns as evidence for the wave-like nature of matter expressed as the de Broglie wavelength = h/p; Momentum of A Photon In the Photon model, photons have energy like a particle, can a photon have momentum? Maxwell had said that an electromagnetic wave which was ca ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.