Solutions
... answer in terms the electrostatic constant, k, fundamental charge, e, and the given quantities, ma and v . ...
... answer in terms the electrostatic constant, k, fundamental charge, e, and the given quantities, ma and v . ...
6.2
... Normally, atoms have equal numbers of electrons and protons, so the net (overall) charge on a material is zero. However, when two materials are rubbed together, electrons may be transferred from one to the other. One material ends up with more electrons than normal and the other with less. So ...
... Normally, atoms have equal numbers of electrons and protons, so the net (overall) charge on a material is zero. However, when two materials are rubbed together, electrons may be transferred from one to the other. One material ends up with more electrons than normal and the other with less. So ...
energy diagrams
... forces can draw energy diagrams • Equilibrium points – Motion will move “around” the equilibrium – If placed there with no energy, will just stay (no force) ...
... forces can draw energy diagrams • Equilibrium points – Motion will move “around” the equilibrium – If placed there with no energy, will just stay (no force) ...
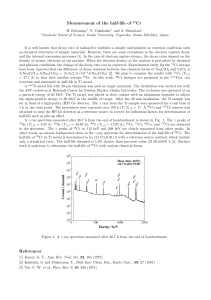
Measurement of the half-life of
... density of atomic electrons at the nucleus. When the electron density at the nucleus is perturbed by chemical and physical conditions, the change of the decay rate can be expected. Experimental study for the 51 Cr isotope have been reported that the difference of decay constant between two chemical f ...
... density of atomic electrons at the nucleus. When the electron density at the nucleus is perturbed by chemical and physical conditions, the change of the decay rate can be expected. Experimental study for the 51 Cr isotope have been reported that the difference of decay constant between two chemical f ...
GENERAL CHEMISTRY SECTION I: ATOMIC THEORY
... The photoelectric effect states that if you shine light on a metal, at a certain υ, electrons are emitted. A classical model for the photoelectric effect could not be verified experimentally – it predicts that there is no relationship between the intensity of the incident light and the energy of the ...
... The photoelectric effect states that if you shine light on a metal, at a certain υ, electrons are emitted. A classical model for the photoelectric effect could not be verified experimentally – it predicts that there is no relationship between the intensity of the incident light and the energy of the ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... isotopic substitution method to estimate bond length? 12 a. Mention in few lines, the best way of studying H2 molecule by spectroscopic technique? 12 b. Explain the theory of pure rotational Raman spectra of a linear molecule (2+ 5.5) 13 Write a note on Dissociation and energy and dissociation produ ...
... isotopic substitution method to estimate bond length? 12 a. Mention in few lines, the best way of studying H2 molecule by spectroscopic technique? 12 b. Explain the theory of pure rotational Raman spectra of a linear molecule (2+ 5.5) 13 Write a note on Dissociation and energy and dissociation produ ...
Physical Setting/Chemistry Examination
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 and 67 on the information below. In 1897, J. J. Thomson demonstrated in an experiment that cathode rays were deflected by an electric field. This suggested that cathode rays were composed of ...
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 and 67 on the information below. In 1897, J. J. Thomson demonstrated in an experiment that cathode rays were deflected by an electric field. This suggested that cathode rays were composed of ...
Bonding Web Practice Trupia - Trupia
... Bromine is the only liquid nonmetallic element at room temperature. It is a heavy, mobile, reddish-brown liquid, volatilizing readily at room temperature to a red vapor with a strong disagreeable odor, resembling chlorine, and having a very irritating effect on the eyes and throat; it is readily sol ...
... Bromine is the only liquid nonmetallic element at room temperature. It is a heavy, mobile, reddish-brown liquid, volatilizing readily at room temperature to a red vapor with a strong disagreeable odor, resembling chlorine, and having a very irritating effect on the eyes and throat; it is readily sol ...
File - Septor CORPORATION
... Nanosystems Group, The MITRE Corporation, McLean, Virginia 22102, USA (The scaling of the capacitance with radius is explored in detail for neutral atoms, and it is found that they behave much like macroscopic spherical capacitors. The quantum capacitances of atoms scale as a linear function of the ...
... Nanosystems Group, The MITRE Corporation, McLean, Virginia 22102, USA (The scaling of the capacitance with radius is explored in detail for neutral atoms, and it is found that they behave much like macroscopic spherical capacitors. The quantum capacitances of atoms scale as a linear function of the ...
Lesson01
... • Note that when the B state dissociates, one of the two atomic fragments is excited. One atom is left in the ground state (3P) and the other in an excited state (1D). • Some fragmentation occurs in the B→X (Schumann-Runge) system before the dissociation limit. This occurs because a repulsive state ...
... • Note that when the B state dissociates, one of the two atomic fragments is excited. One atom is left in the ground state (3P) and the other in an excited state (1D). • Some fragmentation occurs in the B→X (Schumann-Runge) system before the dissociation limit. This occurs because a repulsive state ...
Time of the Energy Emission in the Hydrogen Atom and Its
... Heisenberg strongly criticized the Bohr atomic model as useless because it applied the unobserved elements of the atomic structure like the electron orbits; see e.g. [26]-[28]. Nevertheless the combined orbital parameters, like the orbit radius or orbit length and the time period of the electron cir ...
... Heisenberg strongly criticized the Bohr atomic model as useless because it applied the unobserved elements of the atomic structure like the electron orbits; see e.g. [26]-[28]. Nevertheless the combined orbital parameters, like the orbit radius or orbit length and the time period of the electron cir ...
Three-body recombination with mixed sign light particles
... effort. Also, it seems likely that any future experiments that involve mixed sign light species will need to confine them by a magnetic field. 2. Numerical method To simplify the discussion in this section, I will use the word lepton to refer to the light particle when it could be an electron or pos ...
... effort. Also, it seems likely that any future experiments that involve mixed sign light species will need to confine them by a magnetic field. 2. Numerical method To simplify the discussion in this section, I will use the word lepton to refer to the light particle when it could be an electron or pos ...
X1-1 - murov.info
... 13. Every time a problem is solved you should ask if the conversion factors used and the answer make sense. The ground crew assumed the density of the fuel to be 1.77 kg/L. Consider the density of water and of fuel (Are fuels such as gasoline more or less dense than water?). Does the conversion fact ...
... 13. Every time a problem is solved you should ask if the conversion factors used and the answer make sense. The ground crew assumed the density of the fuel to be 1.77 kg/L. Consider the density of water and of fuel (Are fuels such as gasoline more or less dense than water?). Does the conversion fact ...
ppt - Yale University
... sample. Dr. Lauterbur realized that if a non-uniform magnetic field were used, then the radio signals would come from just one slice of the sample, allowing a two-dimensional image to be created. i.e. one particular frequency The nuclear magnetic resonance machine at SUNY was shared among the chemis ...
... sample. Dr. Lauterbur realized that if a non-uniform magnetic field were used, then the radio signals would come from just one slice of the sample, allowing a two-dimensional image to be created. i.e. one particular frequency The nuclear magnetic resonance machine at SUNY was shared among the chemis ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.