noble gases
... 43. The oxidation state of chromium in the final product formed by the reaction between KI and acidified potassium dichromate solution is a. +4b. +6 c. +2 d. +3 Ans : d K2Cr2O7 is reduced to chromic sulphate, Cr2(SO4)3 in which chromium is in +3 state Vikasana - CET 2012 ...
... 43. The oxidation state of chromium in the final product formed by the reaction between KI and acidified potassium dichromate solution is a. +4b. +6 c. +2 d. +3 Ans : d K2Cr2O7 is reduced to chromic sulphate, Cr2(SO4)3 in which chromium is in +3 state Vikasana - CET 2012 ...
Rydberg Atoms for Quantum Information - Deep Blue
... Doctoral Committee: Professor Professor Professor Professor Assistant ...
... Doctoral Committee: Professor Professor Professor Professor Assistant ...
Heisenberg Uncertainty Principle
... Bohr’s theory. It was first developed using noncommuting algebra and then by matrices. ...
... Bohr’s theory. It was first developed using noncommuting algebra and then by matrices. ...
Ions
... Nonmetals, Group 5A (15), Group 6A (16), and Group 7A (17) • have high ionization energies, they don’t tend to lose electrons but instead gain electrons • readily gain one or more valence electrons to form ions with a negative charge. • gain electrons until they have the same number of valence elect ...
... Nonmetals, Group 5A (15), Group 6A (16), and Group 7A (17) • have high ionization energies, they don’t tend to lose electrons but instead gain electrons • readily gain one or more valence electrons to form ions with a negative charge. • gain electrons until they have the same number of valence elect ...
Chapter 6 Ionic and Molecular Compounds
... Sodium atoms in Group 1A (1) are neutral, with 11 electrons and 11 protons, they • lose one electron to have the same number of valence electrons as neon and a filled energy level. • will form an ion with 10 electrons, 11 protons, and an ionic charge of 1+: Na+. ...
... Sodium atoms in Group 1A (1) are neutral, with 11 electrons and 11 protons, they • lose one electron to have the same number of valence electrons as neon and a filled energy level. • will form an ion with 10 electrons, 11 protons, and an ionic charge of 1+: Na+. ...
The ion-optical design of the MARA recoil separator and absolute
... In this thesis work, the use of two complementary recoil separators for studies of nuclear structure via fusion-evaporation reactions are discussed. The design and the main ion-optical properties of the vacuum-mode recoil-mass separator MARA, intended for studies of nuclei with N ≈ Z close to the pr ...
... In this thesis work, the use of two complementary recoil separators for studies of nuclear structure via fusion-evaporation reactions are discussed. The design and the main ion-optical properties of the vacuum-mode recoil-mass separator MARA, intended for studies of nuclei with N ≈ Z close to the pr ...
Exercises - RACHEL
... Some ideas in science gain the status of theories. A scientific theory is a broad explanation that is widely accepted because it is supported by a great deal of evidence. An example is the kinetic theory of matter. According to this theory, all matter consists of tiny particles that are in constant ...
... Some ideas in science gain the status of theories. A scientific theory is a broad explanation that is widely accepted because it is supported by a great deal of evidence. An example is the kinetic theory of matter. According to this theory, all matter consists of tiny particles that are in constant ...
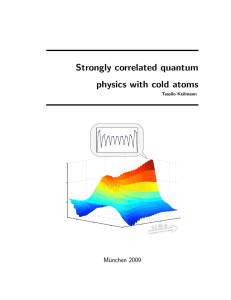
Strongly correlated quantum physics with cold atoms - Max
... The first realization of Bose-Einstein condensates (BEC) in 1995 [1, 2] opened up new pathways in ultracold atomic physics and provided unique opportunities to explore quantum phenomena associated with weak interactions. Many of the early experiments on BECs can indeed be well explained by mean fiel ...
... The first realization of Bose-Einstein condensates (BEC) in 1995 [1, 2] opened up new pathways in ultracold atomic physics and provided unique opportunities to explore quantum phenomena associated with weak interactions. Many of the early experiments on BECs can indeed be well explained by mean fiel ...
Effect of Electron–Electron Interaction on Spin Relaxation of Charge
... mean electron quasimomentum). Indeed, it does not matter whether a change in k (and the corresponding change in the axis of Larmor precession) is due to the scattering by a static defect or a phonon, or due to cyclotron motion of free carriers in magnetic field [11, 23], or it is caused by a collisi ...
... mean electron quasimomentum). Indeed, it does not matter whether a change in k (and the corresponding change in the axis of Larmor precession) is due to the scattering by a static defect or a phonon, or due to cyclotron motion of free carriers in magnetic field [11, 23], or it is caused by a collisi ...
1fp-lecture-notes-electronic-2015
... • 14: Gravitation (and Electromagnetism) – Newton’s law, gravitational potential energy, Kepler’s laws ...
... • 14: Gravitation (and Electromagnetism) – Newton’s law, gravitational potential energy, Kepler’s laws ...
introduction - TestBankTop
... that part of the calculation is determined by the lowest number of digits to the right of the decimal point in any of the original numbers. For the division part of the calculation, the number of significant figures in the answer is determined by the number having the smallest number of significant ...
... that part of the calculation is determined by the lowest number of digits to the right of the decimal point in any of the original numbers. For the division part of the calculation, the number of significant figures in the answer is determined by the number having the smallest number of significant ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.