Creation and Annihilation Operators
... Note that states corresponding to different numbers of particles are orthogonal to each other. E.g., any state in the two-particle subspace H2S is orthogonal to any state in H1S . ◦ Obviously, HFS can contain linear combinations of states with different numbers of particles. While this may at first ...
... Note that states corresponding to different numbers of particles are orthogonal to each other. E.g., any state in the two-particle subspace H2S is orthogonal to any state in H1S . ◦ Obviously, HFS can contain linear combinations of states with different numbers of particles. While this may at first ...
2012 General Chemistry I
... Quantum mechanical description of the distribution of electrons in bonds Valence electrons are localized either between pairs of atoms or on atoms as lone pairs. 1) Hybridization of atomic valence orbitals with proper symmetry that are localized between pairs of atoms. 2) Placing valence electrons i ...
... Quantum mechanical description of the distribution of electrons in bonds Valence electrons are localized either between pairs of atoms or on atoms as lone pairs. 1) Hybridization of atomic valence orbitals with proper symmetry that are localized between pairs of atoms. 2) Placing valence electrons i ...
Homework - PHA Science
... produce nitrogen monoxide gas and liquid water. Solid lithium reacts with nitrogen gas to produce solid lithium nitride. Nitroglycerin (C3H5N3O9) decomposes explosively to produce nitrogen gas, carbon dioxide gas, water vapor and oxygen gas. Carbon dioxide gas reacts with potassium hydroxide to prod ...
... produce nitrogen monoxide gas and liquid water. Solid lithium reacts with nitrogen gas to produce solid lithium nitride. Nitroglycerin (C3H5N3O9) decomposes explosively to produce nitrogen gas, carbon dioxide gas, water vapor and oxygen gas. Carbon dioxide gas reacts with potassium hydroxide to prod ...
chapter 4
... sort of pattern do you think you will observed? It’s the interference pattern that are in fact observed in experiments At the source the electron is being emitted as particle and is experimentally detected as a electron which is absorbed by an individual atom in the fluorescent plate In between, we ...
... sort of pattern do you think you will observed? It’s the interference pattern that are in fact observed in experiments At the source the electron is being emitted as particle and is experimentally detected as a electron which is absorbed by an individual atom in the fluorescent plate In between, we ...
shp_05 - Columbia University
... If elementary particles like the electron are actually little spinning spheres of charge, why should their spins be quantized in magnitude and direction? Classically, there is no way to explain this behavior. In 1925, S. Goudsmidt and G. Uhlenbeck realized that the classical model just cannot apply. ...
... If elementary particles like the electron are actually little spinning spheres of charge, why should their spins be quantized in magnitude and direction? Classically, there is no way to explain this behavior. In 1925, S. Goudsmidt and G. Uhlenbeck realized that the classical model just cannot apply. ...
Chapter 4 Arrangements of Electrons in Atoms
... with period 3: 1. Find the period the element in question is in. 2. Locate the closest noble gas (must have fewer electrons than the element in question). 3. Write the symbol of the noble gas in brackets (This represents ‘x’ number of electrons). 4. Continue the notation with the principal energy le ...
... with period 3: 1. Find the period the element in question is in. 2. Locate the closest noble gas (must have fewer electrons than the element in question). 3. Write the symbol of the noble gas in brackets (This represents ‘x’ number of electrons). 4. Continue the notation with the principal energy le ...
IB1 Introduction to Ch
... Density is an intensive property. It is constant for most solids and liquids, but it depends on the pressure and temperature for a gas ...
... Density is an intensive property. It is constant for most solids and liquids, but it depends on the pressure and temperature for a gas ...
Ch16_2008
... electric field is the direction of the force felt by a positive charge •If there are two or more charges creating the field then the field at any point is the vector sum of the fields created by each of the charges •The test charge does not contribute to the field and it is too weak to cause any of ...
... electric field is the direction of the force felt by a positive charge •If there are two or more charges creating the field then the field at any point is the vector sum of the fields created by each of the charges •The test charge does not contribute to the field and it is too weak to cause any of ...
Chemical Reactions and The Mole
... Generally, you will work with quantities of atoms greater than one. A new unit was developed from work by the Italian Chemist Lorenzo Romano Amedeo Carlo Avogadro. The unit is named the mole or Avogadro’s number. This unit is nothing more than a number, a very big number. The mole works no different ...
... Generally, you will work with quantities of atoms greater than one. A new unit was developed from work by the Italian Chemist Lorenzo Romano Amedeo Carlo Avogadro. The unit is named the mole or Avogadro’s number. This unit is nothing more than a number, a very big number. The mole works no different ...
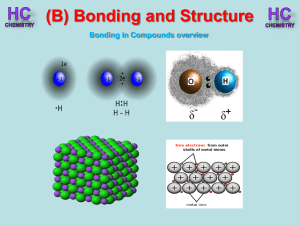
Lesson 1 - Bonding in compounds overview
... Silicon and oxygen make up nearly 75% of the Earth’s crust. They are therefore the most common elements in the Earth’s crust. They combine together to make a covalent network compound called silicon dioxide. This is usually found in the form of sand or quartz. Each Si atom is bonded to 4 O atoms, an ...
... Silicon and oxygen make up nearly 75% of the Earth’s crust. They are therefore the most common elements in the Earth’s crust. They combine together to make a covalent network compound called silicon dioxide. This is usually found in the form of sand or quartz. Each Si atom is bonded to 4 O atoms, an ...
Chapter 3 - Significant Figures - Scientific Measurement
... Our test is also different than the other tests. There are basically two parts: Multiple Choice (60 questions – 90 minutes) and Free Response (4 short questions and 3 long questions – 90 minutes). There is no penalty for guessing on the multiple choice questions. Something different though exists fo ...
... Our test is also different than the other tests. There are basically two parts: Multiple Choice (60 questions – 90 minutes) and Free Response (4 short questions and 3 long questions – 90 minutes). There is no penalty for guessing on the multiple choice questions. Something different though exists fo ...
H3AsO4 + 3 I- + 2 H3O+ H3AsO3 + I3- + H2O
... Electron Arrangements in Atoms and Ions Energy increases as n increases (1 < 2 < 3, etc) and within the same value of n, energy increases as the sublevel progresses from letters s p d f. Orbitals within the same sublevel are degenerate, meaning they have the same energy. The energies of s and ...
... Electron Arrangements in Atoms and Ions Energy increases as n increases (1 < 2 < 3, etc) and within the same value of n, energy increases as the sublevel progresses from letters s p d f. Orbitals within the same sublevel are degenerate, meaning they have the same energy. The energies of s and ...
Chapter 8 The Ideal Gas - Department of Physics | Oregon State
... Even before there was a fully accredited quantum mechanics, W. Pauli1 conjectured that only one electron can occupy a single-particle energy eigen-state – a restriction called the Pauli exclusion principle (PEP). The following year, E. Fermi2 and P. Dirac3 further showed that quantum mechanics requi ...
... Even before there was a fully accredited quantum mechanics, W. Pauli1 conjectured that only one electron can occupy a single-particle energy eigen-state – a restriction called the Pauli exclusion principle (PEP). The following year, E. Fermi2 and P. Dirac3 further showed that quantum mechanics requi ...
Is Matter Made of Light? The Transluminal Energy Quantum (TEQ
... carried a fixed (quantized) amount of electric charge and mass J.J. Thompson discovered the electron as a sub-atomic particle in 1897. He measured the charge to mass ratio of the electron. Later he measured the charge of the electron and calculated its mass. He concluded that electrons come from wit ...
... carried a fixed (quantized) amount of electric charge and mass J.J. Thompson discovered the electron as a sub-atomic particle in 1897. He measured the charge to mass ratio of the electron. Later he measured the charge of the electron and calculated its mass. He concluded that electrons come from wit ...
Word
... (b) Either relatively large total mass (due to batteries, etc.) so since a = F / m, the acceleration is poor. Also, maximum power from batteries is less than available from burning petrol in a petrol engine. Both of these factors are likely to change rapidly due to electric car development in light ...
... (b) Either relatively large total mass (due to batteries, etc.) so since a = F / m, the acceleration is poor. Also, maximum power from batteries is less than available from burning petrol in a petrol engine. Both of these factors are likely to change rapidly due to electric car development in light ...
Encyclopedia - KSU Faculty Member websites
... With the development of the quantum theory of atomic and molecular structure by Niels Bohr and others, it became apparent that light and other forms of electromagnetic radiation are emitted and absorbed in connection with energy transitions of the particles of the substance radiating or absorbing th ...
... With the development of the quantum theory of atomic and molecular structure by Niels Bohr and others, it became apparent that light and other forms of electromagnetic radiation are emitted and absorbed in connection with energy transitions of the particles of the substance radiating or absorbing th ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.