The Equivalent Rest-mass of Photon
... besides f = 0 would cancel the energy of the P itself, as we infer from (1). Moreover it is the Quantum Mechanics (QM) itself, through Heisenberg Uncertainty Principle (HUP), to avoid a P with f = 0, since a P which does not oscillate is a motionless P, in this case we would know simultaneously 2 co ...
... besides f = 0 would cancel the energy of the P itself, as we infer from (1). Moreover it is the Quantum Mechanics (QM) itself, through Heisenberg Uncertainty Principle (HUP), to avoid a P with f = 0, since a P which does not oscillate is a motionless P, in this case we would know simultaneously 2 co ...
How do electrons get across nodes? A problem in the
... so along the lines of de Broglie's double solution. They sugaested that auantum particles are embedded in Madelune's h i d and derive the& motion from it in the same way-in which a pollen a a i n executine Brownian motion derives its movement from the surrounding water. They further suggested that, ...
... so along the lines of de Broglie's double solution. They sugaested that auantum particles are embedded in Madelune's h i d and derive the& motion from it in the same way-in which a pollen a a i n executine Brownian motion derives its movement from the surrounding water. They further suggested that, ...
ESO - ENCIGA
... order to be able to predict its behaviour and understand its history. Science is based on systematic experimentation and on observation of natural phenomena to discover facts about them and to formulate laws and principles based on these facts. The organized knowledge that is derived from scientific ...
... order to be able to predict its behaviour and understand its history. Science is based on systematic experimentation and on observation of natural phenomena to discover facts about them and to formulate laws and principles based on these facts. The organized knowledge that is derived from scientific ...
Unit 3 Review Packet
... What is a substance made of only one type of atom? a. A compound b. A solution c. An element d. A mixture ...
... What is a substance made of only one type of atom? a. A compound b. A solution c. An element d. A mixture ...
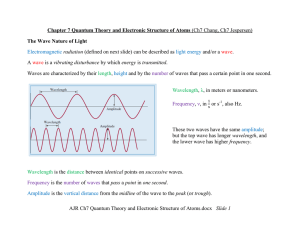
AJR Ch7 Quantum Theory and Electronic Structure of Atoms.docx
... According to classical electromagnetic (wave) theory, this effect can be attributed to the transfer of energy from the light to an electron. An alteration in either the intensity or wavelength of light would induce changes in the rate of emission of electrons from the metal. Furthermore, according ...
... According to classical electromagnetic (wave) theory, this effect can be attributed to the transfer of energy from the light to an electron. An alteration in either the intensity or wavelength of light would induce changes in the rate of emission of electrons from the metal. Furthermore, according ...
True Nature of Potential Energy of a Hydrogen Atom
... includes the electron’s rest mass energy. The energy here is measured on an absolute scale. Because E < E0 in this case, Einstein’s relationship does not apply to an electron in this state. However, the energy of a hydrogen atom, obtained through classical quantum theory or the Schrödinger equation, ...
... includes the electron’s rest mass energy. The energy here is measured on an absolute scale. Because E < E0 in this case, Einstein’s relationship does not apply to an electron in this state. However, the energy of a hydrogen atom, obtained through classical quantum theory or the Schrödinger equation, ...
Physics 30 - Structured Independent Learning
... mass originally at rest ends up travelling at 40 m/s at 30° S of W. What is the resulting velocity of the incoming 50 kg mass? (45.7 m/s at 41o S of E) ...
... mass originally at rest ends up travelling at 40 m/s at 30° S of W. What is the resulting velocity of the incoming 50 kg mass? (45.7 m/s at 41o S of E) ...
Statistical description of systems of particles
... The evolution of a system in a microscopic state is completely deterministic both in quantum and classical mechanics. However, such information cannot be made available for a system with a large number of degrees of freedom. We consider a large number of identical systems (ensemble), all prepared su ...
... The evolution of a system in a microscopic state is completely deterministic both in quantum and classical mechanics. However, such information cannot be made available for a system with a large number of degrees of freedom. We consider a large number of identical systems (ensemble), all prepared su ...
Chapter 1 Introduction: Why are quantum many
... This second sampling is really the crucial step to allow any kind of first-principles quantum simulation of realistic mesoscopic models8 , as the number of variables to keep track of now finally scales proportionally to N . The unavoidable price paid is loss of accuracy. Fortunately most of this pri ...
... This second sampling is really the crucial step to allow any kind of first-principles quantum simulation of realistic mesoscopic models8 , as the number of variables to keep track of now finally scales proportionally to N . The unavoidable price paid is loss of accuracy. Fortunately most of this pri ...
Calculated Electron Dynamics in a Strong Electric Field V 77, N 20
... wonderful to see, in one system, behavior that can be interpreted classically and behavior that must be interpreted quantum mechanically. In this paper we present the results of our calculations that describe the complex dynamics of electron waves in strong, static electric fields. The electron wave ...
... wonderful to see, in one system, behavior that can be interpreted classically and behavior that must be interpreted quantum mechanically. In this paper we present the results of our calculations that describe the complex dynamics of electron waves in strong, static electric fields. The electron wave ...
Worksheet 1 Answer Key from 2010
... 1. Briefly explain what is meant by "wave-particle duality of light" in your own words. Light is neither a wave nor a particle but rather something different which exhibits properties of both waves and particles, sometimes called a "waveicle." 2. What equation corresponds to the wave nature of light ...
... 1. Briefly explain what is meant by "wave-particle duality of light" in your own words. Light is neither a wave nor a particle but rather something different which exhibits properties of both waves and particles, sometimes called a "waveicle." 2. What equation corresponds to the wave nature of light ...
Physics in Ultracold atoms
... ( x1 , x2 ) ( x2 , x1 ), + for boson and - for fermion Therefore, for fermion we have ( x, x) 0, i.e. fermions like to be far away, but bosons do like to be close ! ...
... ( x1 , x2 ) ( x2 , x1 ), + for boson and - for fermion Therefore, for fermion we have ( x, x) 0, i.e. fermions like to be far away, but bosons do like to be close ! ...
JOURNAL DE PHYSIQUE Colloque C2, supplement au n03, Tome 47,
... where KaB = 2.25 exp(-0.13 ~-l IRa - RBI). Because the atomic orbitals lP~ are nonorthogonal. we cannot account for the atomic polarization effects in (3) but only include field effects with the energy shift in (2). In our calculation we keep the geometry of the Fe 4 cluster fixed and vary the nitro ...
... where KaB = 2.25 exp(-0.13 ~-l IRa - RBI). Because the atomic orbitals lP~ are nonorthogonal. we cannot account for the atomic polarization effects in (3) but only include field effects with the energy shift in (2). In our calculation we keep the geometry of the Fe 4 cluster fixed and vary the nitro ...
Chemistry I Exams and Keys Corrected 2016 Season
... 18. Joseph Proust(1754 to 1826) was the chemist to first formally state that: Rejected: because simple memorization. Also, student may not have read about Proust. All full credit. A) When two elements combine with each other to form more than one compound, the weights of one element that combine wi ...
... 18. Joseph Proust(1754 to 1826) was the chemist to first formally state that: Rejected: because simple memorization. Also, student may not have read about Proust. All full credit. A) When two elements combine with each other to form more than one compound, the weights of one element that combine wi ...
Chemistry Unit 5 Test Review The Mole and Balancing Equations
... 1. The percent by mass of oxygen in CO is approximately ___________ 2. Which quantity is equivalent to 39 grams of LiF? 3. What is the mass in grams of 1.00 mole of O 2 gas? 4. What is the total number of moles contained in 115 grams of C 2 H5 OH? 5. What is the total mass in grams of 0.75 mole of S ...
... 1. The percent by mass of oxygen in CO is approximately ___________ 2. Which quantity is equivalent to 39 grams of LiF? 3. What is the mass in grams of 1.00 mole of O 2 gas? 4. What is the total number of moles contained in 115 grams of C 2 H5 OH? 5. What is the total mass in grams of 0.75 mole of S ...
Example 4: A one-electron atom is irradiated with visible light. The
... obtain line spectra unless selective absorption (or emission) was occurring. We would not get selective absorption (or emission) if we did not have discrete energies allowed for the electrons. In the next section we will use the simplest atom, the hydrogen atom, to build up a picture of the electron ...
... obtain line spectra unless selective absorption (or emission) was occurring. We would not get selective absorption (or emission) if we did not have discrete energies allowed for the electrons. In the next section we will use the simplest atom, the hydrogen atom, to build up a picture of the electron ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.