Electron Configuration
... In large families with several children, it is a luxury for each child to have their own room. There is less fussing and fighting if siblings are not forced to share living quarters. The entire household experiences a lower, less frazzled energy state. Electrons find ...
... In large families with several children, it is a luxury for each child to have their own room. There is less fussing and fighting if siblings are not forced to share living quarters. The entire household experiences a lower, less frazzled energy state. Electrons find ...
Topic #4 Notes
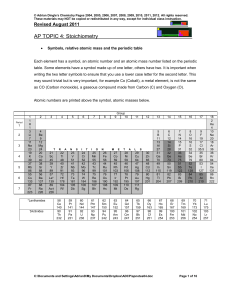
... Average relative atomic masses are the mass numbers recorded on the periodic table. The relative masses of atoms shown on the periodic table can be used to determine the relative masses of molecules and ions by simple summation. Relative Molecular Mass (RMM) or Molar Mass - Found by adding all of th ...
... Average relative atomic masses are the mass numbers recorded on the periodic table. The relative masses of atoms shown on the periodic table can be used to determine the relative masses of molecules and ions by simple summation. Relative Molecular Mass (RMM) or Molar Mass - Found by adding all of th ...
Section 8-2
... • Sharing valence electrons with other atoms also results in noble-gas electron configurations. • The chemical bond that results from sharing electrons is a covalent bond. ...
... • Sharing valence electrons with other atoms also results in noble-gas electron configurations. • The chemical bond that results from sharing electrons is a covalent bond. ...
A Study of Matter
... • Boiling point- liquid turns to a gas (water to water vapor) • Condensation- where a gas turns to a liquid (the sweating on a glass that is colder than it’s environment) • Sublimation point- temperature at which a solid changes directly to a gas without first changing into a liquid. (dry ice) ...
... • Boiling point- liquid turns to a gas (water to water vapor) • Condensation- where a gas turns to a liquid (the sweating on a glass that is colder than it’s environment) • Sublimation point- temperature at which a solid changes directly to a gas without first changing into a liquid. (dry ice) ...
Principles of Chemistry - EPS School Projects - Heriot
... equations using half reactions in acidic and basic solutions; the mole and determination of molecular formula. Atomic Structure – the Rutherford model; the wave-particle duality of light; atomic spectroscopy, the Bohr model; quantum mechanics; Schrödinger wave equation; atomic orbitals; H-like and m ...
... equations using half reactions in acidic and basic solutions; the mole and determination of molecular formula. Atomic Structure – the Rutherford model; the wave-particle duality of light; atomic spectroscopy, the Bohr model; quantum mechanics; Schrödinger wave equation; atomic orbitals; H-like and m ...
L14special - Particle Physics and Particle Astrophysics
... the rotational motion kept them apart. In the model the electron can exist at any radius so long as the rotational velocity increases to compensate. ...
... the rotational motion kept them apart. In the model the electron can exist at any radius so long as the rotational velocity increases to compensate. ...
Basic Constituents of Matter and their Interactions
... precise and detailed investigation. This is because one can tune the e+ e− energy to a desired particle mass (e.g. MZ ), which cannot evidently be done with the quark-antiquark energy. Thus one could produce about a million of Z → e+ e− events per year at LEP while the annual yield of such events at ...
... precise and detailed investigation. This is because one can tune the e+ e− energy to a desired particle mass (e.g. MZ ), which cannot evidently be done with the quark-antiquark energy. Thus one could produce about a million of Z → e+ e− events per year at LEP while the annual yield of such events at ...
Gedanken and real experiments in modern physics - IPN-Kiel
... angle the measurements exhibits some non-trivial correlations. The existence of such correlation worried Einstein, it seemed to him that information about the outcome of Alice’s measurement must be somehow transferred to Bob’s particle, otherwise he could not explain the correlations. These argument ...
... angle the measurements exhibits some non-trivial correlations. The existence of such correlation worried Einstein, it seemed to him that information about the outcome of Alice’s measurement must be somehow transferred to Bob’s particle, otherwise he could not explain the correlations. These argument ...
PPT_W07D1_mac
... Total Force on a System of N Particles is the External Force The total force on a system of particles is the sum of the total external and total internal forces. Since the total internal force is zero ...
... Total Force on a System of N Particles is the External Force The total force on a system of particles is the sum of the total external and total internal forces. Since the total internal force is zero ...
Chemistry - Beachwood City Schools
... d) the formation of a precipitate (formation of a solid from mixing solutions) which represents the formation of an insoluble substance from soluble substances. 7. a) chemical e) chemical ...
... d) the formation of a precipitate (formation of a solid from mixing solutions) which represents the formation of an insoluble substance from soluble substances. 7. a) chemical e) chemical ...
FINAL EXAM REVIEW PROBLEMS
... a. Suppose 6.71 x 103 g of titanium (IV) chloride is reacted with 2.45 x 10 3 g of oxygen. Calculate the maximum mass of titanium (IV) oxide that can form. b. If the percent yield of TiO2 is 75%, what mass was actually produced? ...
... a. Suppose 6.71 x 103 g of titanium (IV) chloride is reacted with 2.45 x 10 3 g of oxygen. Calculate the maximum mass of titanium (IV) oxide that can form. b. If the percent yield of TiO2 is 75%, what mass was actually produced? ...
a, c - Career Launcher
... Class Exercise - 9 A small block of mass = 0.500 kg is released from rest at the top of a curved frictionless wedge of mass = 3 kg, which sits on a frictionless horizontal surface. When it leaves the wedge the block’s velocity is 4 m/s to the right, (i) what is the velocity of the wedge after the b ...
... Class Exercise - 9 A small block of mass = 0.500 kg is released from rest at the top of a curved frictionless wedge of mass = 3 kg, which sits on a frictionless horizontal surface. When it leaves the wedge the block’s velocity is 4 m/s to the right, (i) what is the velocity of the wedge after the b ...
H2 PHYSICS SET B PAPER 1 THE PHYSICS CAFE
... Penelope measures the mass and speed of a glider. The percentage uncertainty in her measurement of the mass is 3% and in the measurement of the speed is 10%. Her calculated value of the kinetic energy of the glider will have an uncertainty of A ...
... Penelope measures the mass and speed of a glider. The percentage uncertainty in her measurement of the mass is 3% and in the measurement of the speed is 10%. Her calculated value of the kinetic energy of the glider will have an uncertainty of A ...
Covalent bonding
... Drawing Lewis structures of polyatomics e.g., draw the Lewis structures of CO2, PCl3 & ClO3-Sum the valence e- from all atoms (use group number) -Write the element symbols - show how they are ...
... Drawing Lewis structures of polyatomics e.g., draw the Lewis structures of CO2, PCl3 & ClO3-Sum the valence e- from all atoms (use group number) -Write the element symbols - show how they are ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.