Electrofreezing of confined water
... Svishchev and Kusalik12,13 studied electrofreezing of supercooled liquid water. They found that for a field with strength of 5 V/nm TIP4P water, at 250 K, undergoes crystallization to cubic ice. They also found at temperatures of 225–240 K and at 3–5 kbar that the homogenous electric field induces t ...
... Svishchev and Kusalik12,13 studied electrofreezing of supercooled liquid water. They found that for a field with strength of 5 V/nm TIP4P water, at 250 K, undergoes crystallization to cubic ice. They also found at temperatures of 225–240 K and at 3–5 kbar that the homogenous electric field induces t ...
Entropy and the Shelf Model: A Quantum Physical Approach to a
... • The shelves cannot be fixed at any height, only distinct positions are allowed depending on the substance or system. This corresponds to the quantum physical eigenvalues. • A smallest quantum leap exists. • The lowest shelf may be fixed at different heights.1 • Different widths represent different ...
... • The shelves cannot be fixed at any height, only distinct positions are allowed depending on the substance or system. This corresponds to the quantum physical eigenvalues. • A smallest quantum leap exists. • The lowest shelf may be fixed at different heights.1 • Different widths represent different ...
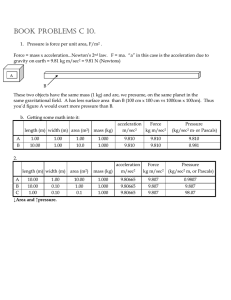
book problems c 10.
... and molecules. The "atoms" of nitrogen and oxygen are in reality "molecules" containing two atoms each. Thus two molecules of hydrogen can combine with one molecule of oxygen to produce two molecules of water. Avogadro suggested that equal volumes of all gases at the same temperature and pressure co ...
... and molecules. The "atoms" of nitrogen and oxygen are in reality "molecules" containing two atoms each. Thus two molecules of hydrogen can combine with one molecule of oxygen to produce two molecules of water. Avogadro suggested that equal volumes of all gases at the same temperature and pressure co ...
Pretest 1
... proton (+1), neutron (neutral) and electron (1) proton (1), neutron (+1) and electron (neutral) proton (+1), neutron (1) and electron (neutral) proton (neutral), neutron (+1) and electron (1) proton (1), neutron (neutral) and electron (+1) ...
... proton (+1), neutron (neutral) and electron (1) proton (1), neutron (+1) and electron (neutral) proton (+1), neutron (1) and electron (neutral) proton (neutral), neutron (+1) and electron (1) proton (1), neutron (neutral) and electron (+1) ...
Spectroscopy of electron ± electron scattering in a 2DEG
... occur in the backscattering direction and are quite small, so that they will be difficult to detect experimentally. For a comparison with experiments we therefore focus on angles a < 1 (rad), where the small-angular scattering peak should provide a clear token of specific 2D phenomena. Another intri ...
... occur in the backscattering direction and are quite small, so that they will be difficult to detect experimentally. For a comparison with experiments we therefore focus on angles a < 1 (rad), where the small-angular scattering peak should provide a clear token of specific 2D phenomena. Another intri ...
Emergent Properties of Discretized Wave
... Conservation laws that characterize the continuous equation will, to high accuracy, be obeyed by the discretized model. These laws will impose constraints on what particle transformations can occur. Many transformations can be expected to start to occur that cannot be completed because of the conser ...
... Conservation laws that characterize the continuous equation will, to high accuracy, be obeyed by the discretized model. These laws will impose constraints on what particle transformations can occur. Many transformations can be expected to start to occur that cannot be completed because of the conser ...
down
... 12.2 The molecular wave function for ground-state H2+ The relative energies of 2 H atoms 2 H atoms : more stable 2624 kJ/mol than 4 separated charges H2 molecule : more stable 436 kJ/mol than infinitely separated 2 H → bond energy is small part of total energy charge distribution of molecule : not ...
... 12.2 The molecular wave function for ground-state H2+ The relative energies of 2 H atoms 2 H atoms : more stable 2624 kJ/mol than 4 separated charges H2 molecule : more stable 436 kJ/mol than infinitely separated 2 H → bond energy is small part of total energy charge distribution of molecule : not ...
2013 us national chemistry olympiad
... d. Nuclear magnetic resonance (NMR) spectroscopy can be used to distinguish between atoms in different environments in a molecule. State and account for the number of unique F-19 signals if 19F NMR were carried out on SF3Cl at i. a low temperature (e.g. –80 °C) ii. room temperature in solution. e. W ...
... d. Nuclear magnetic resonance (NMR) spectroscopy can be used to distinguish between atoms in different environments in a molecule. State and account for the number of unique F-19 signals if 19F NMR were carried out on SF3Cl at i. a low temperature (e.g. –80 °C) ii. room temperature in solution. e. W ...
Chemistry
... Collect and use information on the Periodic Table, including atomic number, atomic mass, family designation, period number, classification of element (metal, nonmetal, semimetal, or metalloid), and the state of the element at room temperature. Identify regions of the periodic table including alkali ...
... Collect and use information on the Periodic Table, including atomic number, atomic mass, family designation, period number, classification of element (metal, nonmetal, semimetal, or metalloid), and the state of the element at room temperature. Identify regions of the periodic table including alkali ...
spin
... Certain quantities are taken into account at the beginning so one works with (i) the final mass --- absorb all mass renormalisations (ii) the final interaction or charge---absorb all charge renormalisations (iii) the final field---absorb all field renormalisations ...
... Certain quantities are taken into account at the beginning so one works with (i) the final mass --- absorb all mass renormalisations (ii) the final interaction or charge---absorb all charge renormalisations (iii) the final field---absorb all field renormalisations ...
Copyright c 2016 by Robert G. Littlejohn Physics 221A Fall 2016
... equation, we look for a complete set of commuting observables for the Hamiltonian (19). In the following we put hats on the operators (p̂i , v̂i , Q̂i , P̂i , X̂, Ŷ ) to distinguish from the corresponding c-numbers (pi , vi , Qi , Pi , X, Y ), where i = 1, 2, 3. For other operators this distinction ...
... equation, we look for a complete set of commuting observables for the Hamiltonian (19). In the following we put hats on the operators (p̂i , v̂i , Q̂i , P̂i , X̂, Ŷ ) to distinguish from the corresponding c-numbers (pi , vi , Qi , Pi , X, Y ), where i = 1, 2, 3. For other operators this distinction ...
Unit G484 - Candidate style answer
... collision the forces acting on the two objects are equal but opposite. The collision time is the same for both so the impulses on the objects are equal and opposite. Impulse = change in momentum so there is no change in momentum ...
... collision the forces acting on the two objects are equal but opposite. The collision time is the same for both so the impulses on the objects are equal and opposite. Impulse = change in momentum so there is no change in momentum ...
Molecules and Ions
... Ionic formulas ALWAYS have a ZERO net charge – i.e. the (+) and (-) ionic charges in ANY formula cancel. If the above rule is followed, the ionic compound must exist and is probably sitting on a shelf in the chemistry stock room! Task: Construct and name as many ionic compounds as possible from the ...
... Ionic formulas ALWAYS have a ZERO net charge – i.e. the (+) and (-) ionic charges in ANY formula cancel. If the above rule is followed, the ionic compound must exist and is probably sitting on a shelf in the chemistry stock room! Task: Construct and name as many ionic compounds as possible from the ...
DYNAMICS AND INFORMATION (Published by Uspekhi
... scattering of quantum particles by macroscopic bodies with subsequent collapse of wave functions of the particles are used to demonstrate how the macroscopic bodies acquire classical properties. To wit, the collapses of wave functions of scattered particles cause collapses of wave functions of macro ...
... scattering of quantum particles by macroscopic bodies with subsequent collapse of wave functions of the particles are used to demonstrate how the macroscopic bodies acquire classical properties. To wit, the collapses of wave functions of scattered particles cause collapses of wave functions of macro ...
Molecules and Ions
... Ionic formulas ALWAYS have a ZERO net charge – i.e. the (+) and (-) ionic charges in ANY formula cancel. If the above rule is followed, the ionic compound must exist and is probably sitting on a shelf in the chemistry stock room! Task: Construct and name as many ionic compounds as possible from the ...
... Ionic formulas ALWAYS have a ZERO net charge – i.e. the (+) and (-) ionic charges in ANY formula cancel. If the above rule is followed, the ionic compound must exist and is probably sitting on a shelf in the chemistry stock room! Task: Construct and name as many ionic compounds as possible from the ...
Power point types of chemical rxn
... 1. Elements that form ionic compounds: Magnesium metal reacts with oxygen gas to form magnesium oxide. • 2Mg + O2 2MgO 2. Elements that form covalent compounds: Nitrogen gas and oxygen gas join to form dinitrogen monoxide. • 2N2 + O2 2N2O SYNTHESIS REACTION (iron + sulphur): http://www.youtube.c ...
... 1. Elements that form ionic compounds: Magnesium metal reacts with oxygen gas to form magnesium oxide. • 2Mg + O2 2MgO 2. Elements that form covalent compounds: Nitrogen gas and oxygen gas join to form dinitrogen monoxide. • 2N2 + O2 2N2O SYNTHESIS REACTION (iron + sulphur): http://www.youtube.c ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.