PH0008 Quantum Mechanics and Special
... Planck suggests ad hoc that the radiation emitted from the walls must happen in discrete bundles (called quanta) such that E=h . Mathematically this additional effect generates an expression for spectrum that fits data well. • The Planck constant is determined empirically from then existing data • ...
... Planck suggests ad hoc that the radiation emitted from the walls must happen in discrete bundles (called quanta) such that E=h . Mathematically this additional effect generates an expression for spectrum that fits data well. • The Planck constant is determined empirically from then existing data • ...
G25.2666: Quantum Mechanics II
... is a constant of the motion. Recall that J is the total angular momentum. It is often true for spin-dependent Hamiltonians that the total angular momentum is still conserved. Thus, we see that total angular orbital angular momentum, total spin, and total angular momentum are all important quantities ...
... is a constant of the motion. Recall that J is the total angular momentum. It is often true for spin-dependent Hamiltonians that the total angular momentum is still conserved. Thus, we see that total angular orbital angular momentum, total spin, and total angular momentum are all important quantities ...
Coulomb Drag to Measure Electron-Electron Interaction in Bilayer
... Notice that individual layer scattering times are going to disappear from the ratio between E1 and I2. This is immensely important - because we have now related a transport measurement to electron-electron scattering . The effect of disorder has somehow disappeared - at least within the relaxation t ...
... Notice that individual layer scattering times are going to disappear from the ratio between E1 and I2. This is immensely important - because we have now related a transport measurement to electron-electron scattering . The effect of disorder has somehow disappeared - at least within the relaxation t ...
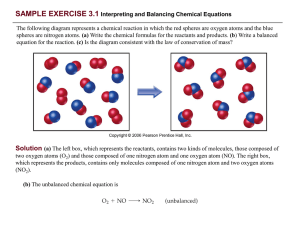
PRACTICE EXERCISE - Needham.K12.ma.us
... Analyze: We are given the number of grams and chemical formula and asked to calculate (a) the number of molecules and (b) the number of O atoms in the sample. (a) Plan: The strategy for determining the number of molecules in a given quantity of a substance is summarized in Figure 3.10. We must conve ...
... Analyze: We are given the number of grams and chemical formula and asked to calculate (a) the number of molecules and (b) the number of O atoms in the sample. (a) Plan: The strategy for determining the number of molecules in a given quantity of a substance is summarized in Figure 3.10. We must conve ...
chemical potential dependence of particle ratios within a
... the experimental data. It is seen that the ratios increase towards unity with an increase in collision energies and with a decrease in the chemical potential. The ratios appear ordered with the strangeness quantum number, i. e., the higher the strangeness quantum number is, the smaller the difference ...
... the experimental data. It is seen that the ratios increase towards unity with an increase in collision energies and with a decrease in the chemical potential. The ratios appear ordered with the strangeness quantum number, i. e., the higher the strangeness quantum number is, the smaller the difference ...
1. Bromine exists naturally as a mixture of bromine
... body during stress and increases the body's metabolic rate. Epinephrine, like many biochemical compounds, is composed of carbon, hydrogen, oxygen, and nitrogen. The percentage composition of the hormone is 56.8% C, 6.56% H, 28.4% O, and 8.28% N. Determine the empirical formula. ...
... body during stress and increases the body's metabolic rate. Epinephrine, like many biochemical compounds, is composed of carbon, hydrogen, oxygen, and nitrogen. The percentage composition of the hormone is 56.8% C, 6.56% H, 28.4% O, and 8.28% N. Determine the empirical formula. ...
F.Y. BSc Notes Interaction of Electromagnetic Radiations with Matter
... transitions occur between energy levels. However, such transition can take place between definite energy levels and not just between any two energy levels. Thus there are certain restrictions on transitions taking place between energy levels which are governed by certain rules called selection rules ...
... transitions occur between energy levels. However, such transition can take place between definite energy levels and not just between any two energy levels. Thus there are certain restrictions on transitions taking place between energy levels which are governed by certain rules called selection rules ...
NUCLEI of ATOMS Vladislav Konovalov Abstract
... Here it is necessary to find out reasons of stability of neutrons in a nucleus conditioning stability and nuclei. As against official notionы new physics explains stability of neutrons in a nucleus to that they are in powerful a gravidynamic field, which one exceeds those values, which one it would ...
... Here it is necessary to find out reasons of stability of neutrons in a nucleus conditioning stability and nuclei. As against official notionы new physics explains stability of neutrons in a nucleus to that they are in powerful a gravidynamic field, which one exceeds those values, which one it would ...
Block 1 - cloudfront.net
... 2. Skeleton equation is an equation that identifies the reactants and products in a chemical reaction by their chemical formula but does not quantify them 3. Coefficients is a multiplicative factor in some term of an expression (or of a series); it is usually a number 4. Balanced equation shows how ...
... 2. Skeleton equation is an equation that identifies the reactants and products in a chemical reaction by their chemical formula but does not quantify them 3. Coefficients is a multiplicative factor in some term of an expression (or of a series); it is usually a number 4. Balanced equation shows how ...
CHEMISTRY - careerpoint.ac.in
... never exist. Therefore, 1 mole of those metals represented by the weight of NA number of such metal atoms and thus, 1 mole of the given substances is equal to gram atomic weight of those metals. (f) Mole concept is based on the application of the law of conservation of atoms, first proposed by Dalto ...
... never exist. Therefore, 1 mole of those metals represented by the weight of NA number of such metal atoms and thus, 1 mole of the given substances is equal to gram atomic weight of those metals. (f) Mole concept is based on the application of the law of conservation of atoms, first proposed by Dalto ...
solution - Seattle Central College
... A short time t2 later, the pendulum is at its lowest position moving to the left. 7). [5 pts]What is the direction of the acceleration vector at t=t2? Express your answer in terms of the unit vectors x̂ and ŷ . The acceleration is in the direction of the net force. At the lowest point, the tension ...
... A short time t2 later, the pendulum is at its lowest position moving to the left. 7). [5 pts]What is the direction of the acceleration vector at t=t2? Express your answer in terms of the unit vectors x̂ and ŷ . The acceleration is in the direction of the net force. At the lowest point, the tension ...
Quantum Information and Randomness - Max-Planck
... physics are not only of philosophical interest but have opened up striking new possibilities of a new quantum information technology. Features such as superposition and entanglement can be exploited to solve certain tasks, which cannot be solved – or at least cannot be solved that efficiently – by pu ...
... physics are not only of philosophical interest but have opened up striking new possibilities of a new quantum information technology. Features such as superposition and entanglement can be exploited to solve certain tasks, which cannot be solved – or at least cannot be solved that efficiently – by pu ...
x - Piazza
... Note that more than one wave function can have the same energy. When more than one wave function has the same energy, those quantum states are said to be degenerate. Degeneracy results from symmetries of the potential energy function that describes the system. A perturbation of the potential energy ...
... Note that more than one wave function can have the same energy. When more than one wave function has the same energy, those quantum states are said to be degenerate. Degeneracy results from symmetries of the potential energy function that describes the system. A perturbation of the potential energy ...
Chemistry II Exams and Answer Keys 2015 Season
... cylinder used to store and transport high pressure gases for scuba divers. When high pressure gases in the scuba tank come in contact with water in the blood stream, these gases dissolve into the blood stream. As a diver swims to the surface, the gases are released. This can cause a very painful con ...
... cylinder used to store and transport high pressure gases for scuba divers. When high pressure gases in the scuba tank come in contact with water in the blood stream, these gases dissolve into the blood stream. As a diver swims to the surface, the gases are released. This can cause a very painful con ...
Some basic concepts of chemistry
... It states that, “Mass is neither created nor destroyed during chemical combination of matter.” Explanation: a. According to Lavoisier, total masses of the reactants before the reaction are found to be same as that of total masses of the products formed after the reaction. b. Eg. AgNO3 + NaCl ⎯→ AgCl ...
... It states that, “Mass is neither created nor destroyed during chemical combination of matter.” Explanation: a. According to Lavoisier, total masses of the reactants before the reaction are found to be same as that of total masses of the products formed after the reaction. b. Eg. AgNO3 + NaCl ⎯→ AgCl ...
Document
... C) The element is located in period 2 and is an alkali metal. D) The element is located in period 2 and is an alkaline earth metal. 3. In the diagram below, the circles numbered 1 to 6 represent a characteristic shared by categories of elements in the periodic table. ...
... C) The element is located in period 2 and is an alkali metal. D) The element is located in period 2 and is an alkaline earth metal. 3. In the diagram below, the circles numbered 1 to 6 represent a characteristic shared by categories of elements in the periodic table. ...
Bell`s experiment with intra- and inter
... 关8–12兴. Our starting point is the single-particle state given in Eq. 共1兲 where the first and second quantum numbers now represent the number of particles in two modes that are localized at two spatially separated and ideally distant locations 共see Fig. 2兲. We refer to such delocalized particles as f ...
... 关8–12兴. Our starting point is the single-particle state given in Eq. 共1兲 where the first and second quantum numbers now represent the number of particles in two modes that are localized at two spatially separated and ideally distant locations 共see Fig. 2兲. We refer to such delocalized particles as f ...
CHAPTER 8: ENERGY FROM ELECTRON TRANSFER
... c. Lead is being oxidized, so lead is the anode. Lead dioxide is being reduced, so it is the cathode. 13. What is meant by the term hybrid car? Answer: In current usage, the term hybrid car refers to the combination of a gasoline engine with a nickel-metal hydride battery, an electric motor, and an ...
... c. Lead is being oxidized, so lead is the anode. Lead dioxide is being reduced, so it is the cathode. 13. What is meant by the term hybrid car? Answer: In current usage, the term hybrid car refers to the combination of a gasoline engine with a nickel-metal hydride battery, an electric motor, and an ...
LHCC - uniud.it
... Spin S - W. Pauli introduced for the 1st time a fourth quantic number the spin - to completely describe the electron state inside the atomic orbitals - No physics meaning was assigned to the spin until 1927, when the experiment of Phipps ad Taylor associated to the spin a magnetic moment of the ele ...
... Spin S - W. Pauli introduced for the 1st time a fourth quantic number the spin - to completely describe the electron state inside the atomic orbitals - No physics meaning was assigned to the spin until 1927, when the experiment of Phipps ad Taylor associated to the spin a magnetic moment of the ele ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.