Electronic Structure of Superheavy Atoms. Revisited.
... more than the critical value Zc = α−1 ' 137, 04, where α is the finite structure constant, is of fundamental importance. The formulation of QED cannot be considered really completed until an exhaustive answer to this question is given. Although nuclei with overcritical charges can hardly be synthesi ...
... more than the critical value Zc = α−1 ' 137, 04, where α is the finite structure constant, is of fundamental importance. The formulation of QED cannot be considered really completed until an exhaustive answer to this question is given. Although nuclei with overcritical charges can hardly be synthesi ...
Practice Questions Section 2
... Write balanced chemical equations for each of the following. Pay close attention to the physical states! Also - you must include the charge when writing ions, otherwise your answer is incorrect. Do not balance these equations using fractions for coefficients. sulfur dioxide gas combines with oxygen ...
... Write balanced chemical equations for each of the following. Pay close attention to the physical states! Also - you must include the charge when writing ions, otherwise your answer is incorrect. Do not balance these equations using fractions for coefficients. sulfur dioxide gas combines with oxygen ...
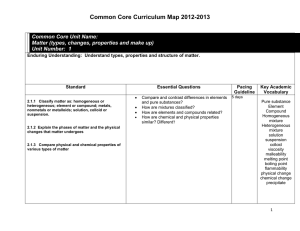
All Units Curriculum Maps
... Standard 3.31 Summarize static and current electricity. 3.32 Explain simple series and parallel circuits in terms of Ohm’s law. 3.3.3 Explain how current is affected by changes in composition, length, temperature and diameter of the ...
... Standard 3.31 Summarize static and current electricity. 3.32 Explain simple series and parallel circuits in terms of Ohm’s law. 3.3.3 Explain how current is affected by changes in composition, length, temperature and diameter of the ...
Moving Lonely Electrons WAVES
... We look at wave functions for moving electrons and describe the probabilities for its location. ...
... We look at wave functions for moving electrons and describe the probabilities for its location. ...
CHAPTER 1 – INTRODUCTION
... Space: is the geometric region occupied by bodies whose positions are described by linear or angular measurements relative to a specific coordinate system. For three dimensional problems, three independent coordinates are needed. For two dimensional problems only two coordinates will be required. T ...
... Space: is the geometric region occupied by bodies whose positions are described by linear or angular measurements relative to a specific coordinate system. For three dimensional problems, three independent coordinates are needed. For two dimensional problems only two coordinates will be required. T ...
SiC @ a-SiO 2
... • model problem limits: cluster of ~2 nm @ ~2.5 (3) nm of matrix larger models • defects and interface chemistry (N,H) a manifold of sligthly different models ...
... • model problem limits: cluster of ~2 nm @ ~2.5 (3) nm of matrix larger models • defects and interface chemistry (N,H) a manifold of sligthly different models ...
Generalized Statistical Approach to the Study of Interatomic Interactions M. E.
... supermolecular density which includes an overlap correction yields much better results than the simple superposition of atomic densities, in particular for the separate energy contributions. As remarked by Nikulin and Tsarev [ 5 ] , for the latter case the total interaction energy improves with resp ...
... supermolecular density which includes an overlap correction yields much better results than the simple superposition of atomic densities, in particular for the separate energy contributions. As remarked by Nikulin and Tsarev [ 5 ] , for the latter case the total interaction energy improves with resp ...
STUDY SHEET – CHEM 1211 LECTURE FINAL EXAM
... Lesson 6 (4 contact hours) Covalent Bonding and Molecular Structure a. The student will draw a correct Lewis symbol for any atom or ion. b. The student will draw correct Lewis structures for molecules that obey the octet rule, for molecules which have multiple bonds, for molecules which are exceptio ...
... Lesson 6 (4 contact hours) Covalent Bonding and Molecular Structure a. The student will draw a correct Lewis symbol for any atom or ion. b. The student will draw correct Lewis structures for molecules that obey the octet rule, for molecules which have multiple bonds, for molecules which are exceptio ...
Wednesday, April 3, 2013
... Example for Two Dimensional Collisions Proton #1 with a speed 3.50x105 m/s collides elastically with proton #2 initially at rest. After the collision, proton #1 moves at an angle of 37o to the horizontal axis and proton #2 deflects at an angle f to the same axis. Find the final speeds of the two pr ...
... Example for Two Dimensional Collisions Proton #1 with a speed 3.50x105 m/s collides elastically with proton #2 initially at rest. After the collision, proton #1 moves at an angle of 37o to the horizontal axis and proton #2 deflects at an angle f to the same axis. Find the final speeds of the two pr ...
Indian National Chemistry Olympiad Theory 2014
... Hydrogen is a renewable source of energy and considered as a fuel of the future. One of the problems of its use is storage and transportation. Research has shown that several metal hydrides act as ‘hydrogen tanks’. Large quantities of hydrogen can be absorbed on them and desorbed when needed through ...
... Hydrogen is a renewable source of energy and considered as a fuel of the future. One of the problems of its use is storage and transportation. Research has shown that several metal hydrides act as ‘hydrogen tanks’. Large quantities of hydrogen can be absorbed on them and desorbed when needed through ...
Stoichiometry Practice
... 1. How many moles of H2 would be required to completely react with O2 to produce 5 moles of water? 5 mol H2 2. H2SO4 + NaOH Na2SO4 + H2O a. Balance this equation b. What mass of H2SO4 would be required to react with 0.75 mol of NaOH? 37g 3. What mass of NO2 is formed when NO reacts with 384 g of O ...
... 1. How many moles of H2 would be required to completely react with O2 to produce 5 moles of water? 5 mol H2 2. H2SO4 + NaOH Na2SO4 + H2O a. Balance this equation b. What mass of H2SO4 would be required to react with 0.75 mol of NaOH? 37g 3. What mass of NO2 is formed when NO reacts with 384 g of O ...
For printing - Mathematical Sciences Publishers
... the measurement problem and the appearance of a classical behavior in a quantum system in the context of the Copenhagen interpretation of quantum mechanics. In a first possible approach, the α-particle is the quantum system under consideration and the gas of the chamber acts as the measurement devic ...
... the measurement problem and the appearance of a classical behavior in a quantum system in the context of the Copenhagen interpretation of quantum mechanics. In a first possible approach, the α-particle is the quantum system under consideration and the gas of the chamber acts as the measurement devic ...
TRANSITION ELEMENTS
... Transition elements refer to those in the d-block of the Periodic Table. The sequence from scandium (Z = 21) to zinc (Z = 30) form what is generally regarded as the first transition series. Virtually ALL the properties of transition elements are related to their electronic structures, in particular ...
... Transition elements refer to those in the d-block of the Periodic Table. The sequence from scandium (Z = 21) to zinc (Z = 30) form what is generally regarded as the first transition series. Virtually ALL the properties of transition elements are related to their electronic structures, in particular ...
hw 10
... labels the total number of excitations of the wave function More precisely n − 1 is the total number of excitations in either the radial or angular directions. • Note: For a general radial potential the energy of the wave depends on wether the excitation is in the angular or radial direction. Thus t ...
... labels the total number of excitations of the wave function More precisely n − 1 is the total number of excitations in either the radial or angular directions. • Note: For a general radial potential the energy of the wave depends on wether the excitation is in the angular or radial direction. Thus t ...
AP Chemistry - Pompton Lakes School District
... whole-group discussions, and small-group work. Science involves using language, both oral and written, as a tool for making thinking public. An atom’s electron configuration, particularly of the outermost electrons, determines how the atom interacts with other atoms. Chemical bonds are the inter ...
... whole-group discussions, and small-group work. Science involves using language, both oral and written, as a tool for making thinking public. An atom’s electron configuration, particularly of the outermost electrons, determines how the atom interacts with other atoms. Chemical bonds are the inter ...
Organic Chemistry Curriculum Map - Belle Vernon Area School District
... CHEM.B.1.2.2 – Apply the law of definite proportions to the classification of elements and compounds as pure substances Standard: 3.2.C.A4 – Predict how combinations of substances can result in physical and/or chemical changes. Anchor: CHEM.B.1.1 – Explain how the mole is a fundamental unit of che ...
... CHEM.B.1.2.2 – Apply the law of definite proportions to the classification of elements and compounds as pure substances Standard: 3.2.C.A4 – Predict how combinations of substances can result in physical and/or chemical changes. Anchor: CHEM.B.1.1 – Explain how the mole is a fundamental unit of che ...
LD5655.V856_1971.I62
... In order to observe transitions between quadrupole enerfzy' levels one can in principle either apply an oscillating electric field, thereby producing an electric field gradients at the nucleus which would interact with the electric quadrupole moment of the nucleus or apply an oscillating magnetic fi ...
... In order to observe transitions between quadrupole enerfzy' levels one can in principle either apply an oscillating electric field, thereby producing an electric field gradients at the nucleus which would interact with the electric quadrupole moment of the nucleus or apply an oscillating magnetic fi ...
Chapter 4 Energy and Stability
... for some function V (x), called the potential energy. (This is the 3D equivalent of F = −V 0 (x) in 1D; in 1D all force fields F (x) are conservative.) But in 3D, not all force R Bfields are conservative, because (as shown in the Vector Calculus course) the value of a line integral A F . dx depends, ...
... for some function V (x), called the potential energy. (This is the 3D equivalent of F = −V 0 (x) in 1D; in 1D all force fields F (x) are conservative.) But in 3D, not all force R Bfields are conservative, because (as shown in the Vector Calculus course) the value of a line integral A F . dx depends, ...
Document
... Water is a good solvent for ionic compounds because it is a polar molecule. The polarity of water results from electron distributions within the molecule. The oxygen atom has an attraction for the hydrogen atoms’ electrons and is therefore partially negative compared to hydrogen. The oxygen atom is ...
... Water is a good solvent for ionic compounds because it is a polar molecule. The polarity of water results from electron distributions within the molecule. The oxygen atom has an attraction for the hydrogen atoms’ electrons and is therefore partially negative compared to hydrogen. The oxygen atom is ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.