Circularly Polarized Near-field Scanning Optical Microscope for
... the photovoltage is understood by the type of the innermost incompressible strip, whether it is Is or I, that separates the bulk and the edge regions [7] as schematically shown in Fig. 6. A spin-split incompressible strip Is with a width wa separates the bulk and the edge regions for 2m − 1 < ν < 2m ...
... the photovoltage is understood by the type of the innermost incompressible strip, whether it is Is or I, that separates the bulk and the edge regions [7] as schematically shown in Fig. 6. A spin-split incompressible strip Is with a width wa separates the bulk and the edge regions for 2m − 1 < ν < 2m ...
9.1-10.5 Organic Chemistry
... Remember Lewis Dot Diagrams from Chem 20?? This means carbon can bond extensively and can bond together to form chains effectively = called Polymerism Carbon covalently bonds by sharing 4 pairs of electrons. These bonds may be single, double or triple, all producing stable compounds Compound ...
... Remember Lewis Dot Diagrams from Chem 20?? This means carbon can bond extensively and can bond together to form chains effectively = called Polymerism Carbon covalently bonds by sharing 4 pairs of electrons. These bonds may be single, double or triple, all producing stable compounds Compound ...
PDF
... design of computer where computations are performed by manipulating single electrons – the ultimate limit in electronic computers. In this problem, we are going to investigate the physics behind such manipulations. Problem Formulation You might recall from your physics class that an electron is neit ...
... design of computer where computations are performed by manipulating single electrons – the ultimate limit in electronic computers. In this problem, we are going to investigate the physics behind such manipulations. Problem Formulation You might recall from your physics class that an electron is neit ...
Interactions and interference in quantum dots : kinks in
... Small circular dots behave much like atoms (hence “artificial atoms”): the circular symmetry causes degeneracy of the orbital levels and so a large spacing between allowed energies. In sharp contrast, there is no degeneracy in irregular dots: the typical single-particle orbital level separation is s ...
... Small circular dots behave much like atoms (hence “artificial atoms”): the circular symmetry causes degeneracy of the orbital levels and so a large spacing between allowed energies. In sharp contrast, there is no degeneracy in irregular dots: the typical single-particle orbital level separation is s ...
Interactions and Interference in Quantum Dots: Kinks in Coulomb
... Small circular dots behave much like atoms (hence “artificial atoms”): the circular symmetry causes degeneracy of the orbital levels and so a large spacing between allowed energies. In sharp contrast, there is no degeneracy in irregular dots: the typical single-particle orbital level separation is s ...
... Small circular dots behave much like atoms (hence “artificial atoms”): the circular symmetry causes degeneracy of the orbital levels and so a large spacing between allowed energies. In sharp contrast, there is no degeneracy in irregular dots: the typical single-particle orbital level separation is s ...
Linear Momentum Test Mr. Kepple
... Two pendulums A and B are aligned side-by-side such that the bobs of each pendulum just barely touch. The mass of each string is negligible and has identical length . Pendulum A has mass while pendulum B has mass . Pendulum A is pulled back away from the equilibrium position to a height 0.10 meters ...
... Two pendulums A and B are aligned side-by-side such that the bobs of each pendulum just barely touch. The mass of each string is negligible and has identical length . Pendulum A has mass while pendulum B has mass . Pendulum A is pulled back away from the equilibrium position to a height 0.10 meters ...
Chapter12
... b. number of molecules - the balanced equation shows that 1 molecule of nitrogen reacts with 3 molecules of hydrogen in order to form 2 molecules of ammonia. The ratio of molecules of N2:H2:NH3 is always 1:3:2. This means that if you could get 10 molecules of nitrogen to react with 30 molecules of h ...
... b. number of molecules - the balanced equation shows that 1 molecule of nitrogen reacts with 3 molecules of hydrogen in order to form 2 molecules of ammonia. The ratio of molecules of N2:H2:NH3 is always 1:3:2. This means that if you could get 10 molecules of nitrogen to react with 30 molecules of h ...
chapter 3 Questions
... copper(II) sulfate pentahydrate (CuSO4.5H2O). When this compound is heated in air above 100° C, it loses the water molecules and also its blue color: CuSO4.5H2O CuSO4 + 5H2O If 9.60 g of CuSO4 are left after heating 15.01 g of the blue compound, calculate the number of moles of H2O originally pres ...
... copper(II) sulfate pentahydrate (CuSO4.5H2O). When this compound is heated in air above 100° C, it loses the water molecules and also its blue color: CuSO4.5H2O CuSO4 + 5H2O If 9.60 g of CuSO4 are left after heating 15.01 g of the blue compound, calculate the number of moles of H2O originally pres ...
Textbook Answer Keys - Mr. Massey`s Chemistry Pages
... 15. Discuss - Offer a considered and balanced review that includes a range of arguments, factors or hypotheses. Opinions or conclusions should be presented clearly and supported by appropriate evidence. [Assessment Objective 3] The Dalton model of the atom as an indivisible particles was changed as ...
... 15. Discuss - Offer a considered and balanced review that includes a range of arguments, factors or hypotheses. Opinions or conclusions should be presented clearly and supported by appropriate evidence. [Assessment Objective 3] The Dalton model of the atom as an indivisible particles was changed as ...
Systems of particles
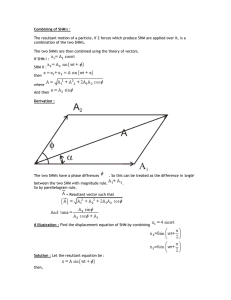
... So far, we have not encountered the concept of momentum except for a single particle. Does it make sense to ask what is the total momentum of two particles? If so, how should it be defined? Since we are free to define the momentum of the system as we wish, we will obviously choose a definition that ...
... So far, we have not encountered the concept of momentum except for a single particle. Does it make sense to ask what is the total momentum of two particles? If so, how should it be defined? Since we are free to define the momentum of the system as we wish, we will obviously choose a definition that ...
File
... abundance of ions often as a percentage. For an element the height of each peak gives the relative isotopic abundance. E.g. 79% are the 24 Mg isotope. • The X axis units are given as “mass/charge” ratio. Since the charge on the ions is mostly +1, you can often assume the x axis is simply the relativ ...
... abundance of ions often as a percentage. For an element the height of each peak gives the relative isotopic abundance. E.g. 79% are the 24 Mg isotope. • The X axis units are given as “mass/charge” ratio. Since the charge on the ions is mostly +1, you can often assume the x axis is simply the relativ ...
Has the Periodic Table Been Successfully Axiomatized?
... represent graphically. I delay a discussion of the main substance of this claim regarding the status of the periodic law. The following section Hettema and Kuiper’s article consists of a brief and generally accurate account of the early historical development of the periodic table. The only importan ...
... represent graphically. I delay a discussion of the main substance of this claim regarding the status of the periodic law. The following section Hettema and Kuiper’s article consists of a brief and generally accurate account of the early historical development of the periodic table. The only importan ...
Discrete Symmetries
... violation in the B-meson sector. • CP violation should also be observable in the D-meson (charm) sector, though this will be a small effect that will be very difficult to measure. • CP violation observed in the K and B mesons is not enough to explain the domination of matter in the universe • With t ...
... violation in the B-meson sector. • CP violation should also be observable in the D-meson (charm) sector, though this will be a small effect that will be very difficult to measure. • CP violation observed in the K and B mesons is not enough to explain the domination of matter in the universe • With t ...
A) 0% B) 20% C) 50% D) 80% E) 100% 1. Naturally occurring boron
... 50. In 1811 Avogadro calculated the formula of camphor by means of elemental chemical analysis and by measuring the density of its vapor. Avogadro found the density to be 3.84 g/L when he made the measurements at 210ºC at 1 atmosphere pressure. Which of the following is the correct formula for cam ...
... 50. In 1811 Avogadro calculated the formula of camphor by means of elemental chemical analysis and by measuring the density of its vapor. Avogadro found the density to be 3.84 g/L when he made the measurements at 210ºC at 1 atmosphere pressure. Which of the following is the correct formula for cam ...
Practice Questions Section 2
... Write balanced chemical equations for each of the following. Pay close attention to the physical states! Also - you must include the charge when writing ions, otherwise your answer is incorrect. Do not balance these equations using fractions for coefficients. sulfur dioxide gas combines with oxygen ...
... Write balanced chemical equations for each of the following. Pay close attention to the physical states! Also - you must include the charge when writing ions, otherwise your answer is incorrect. Do not balance these equations using fractions for coefficients. sulfur dioxide gas combines with oxygen ...
The Concept of Probability in Quantum Mechanics
... From about the beginning of the twentieth century experimental physics amassed an impressive array of strange phenomena which demonstrated the inadequacy of classical physics. The attempts to discover a theoretical structure for the new phenomena led at first to a confusion in which it appeared tha ...
... From about the beginning of the twentieth century experimental physics amassed an impressive array of strange phenomena which demonstrated the inadequacy of classical physics. The attempts to discover a theoretical structure for the new phenomena led at first to a confusion in which it appeared tha ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.