Regular article A valence-bond-based complete-active-space
... contributes to the Pijab elements for the six carbon–carbon p bonds. However, there are six more covalent determinants (coefficient 0.146 each) with parallel spins on neighboring carbon atoms that only contribute to four Pijab elements each. Finally, there are 12 ionic terms (coefficient 0.142 each) tha ...
... contributes to the Pijab elements for the six carbon–carbon p bonds. However, there are six more covalent determinants (coefficient 0.146 each) with parallel spins on neighboring carbon atoms that only contribute to four Pijab elements each. Finally, there are 12 ionic terms (coefficient 0.142 each) tha ...
Nuclear physics - Sciencemadness
... Writing balanced nuclear equations: ................................ 44 Radioactive decay: ............................................................ 45 The decay constant: ...................................................... 45 Binding energy: ................................................... ...
... Writing balanced nuclear equations: ................................ 44 Radioactive decay: ............................................................ 45 The decay constant: ...................................................... 45 Binding energy: ................................................... ...
How do we predict chemical change?
... Recall from Unit 4 that differences in the bond energy of reactants and products are responsible for the absorption or release of energy during a chemical reaction. If the products of a chemical process are made up of molecules with stronger bonds than those present in the molecules of the reactants ...
... Recall from Unit 4 that differences in the bond energy of reactants and products are responsible for the absorption or release of energy during a chemical reaction. If the products of a chemical process are made up of molecules with stronger bonds than those present in the molecules of the reactants ...
Reaction Rates
... The reactants are being turned into products at the same rate as products are being turned into reactants. ...
... The reactants are being turned into products at the same rate as products are being turned into reactants. ...
Lft} (rr
... As per University Order read as first, Credit Semester System was implemented to PG programmes in affiliated Arts and Science Colltges and Self Financing Centres of the University with effect from 2010 admission onwards. The Board of Studies in Chemistry ,vide paper read as second, considered the nu ...
... As per University Order read as first, Credit Semester System was implemented to PG programmes in affiliated Arts and Science Colltges and Self Financing Centres of the University with effect from 2010 admission onwards. The Board of Studies in Chemistry ,vide paper read as second, considered the nu ...
Electrokinetic colloid patterning characterization with a
... FIGURE A3 - Analysis data for experiment 1.................................................................. 76 FIGURE A4 - Analysis data for experiment 8.................................................................. 77 FIGURE A5 - Analysis data for experiment 9.................................. ...
... FIGURE A3 - Analysis data for experiment 1.................................................................. 76 FIGURE A4 - Analysis data for experiment 8.................................................................. 77 FIGURE A5 - Analysis data for experiment 9.................................. ...
Theoretical aspects of Solid State Physics
... of spectrum of Hydrogen atom, or for a value of the fundamental constant e2 /h̄c. Typically, however, it is not so, because of two reasons: first, objects are too complicated in almost all cases and, second, physical laws often have an approximate nature. Therefore, to solve a particular problem, on ...
... of spectrum of Hydrogen atom, or for a value of the fundamental constant e2 /h̄c. Typically, however, it is not so, because of two reasons: first, objects are too complicated in almost all cases and, second, physical laws often have an approximate nature. Therefore, to solve a particular problem, on ...
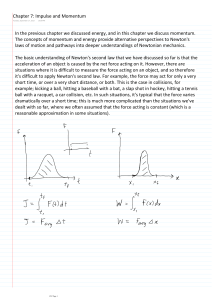
In the previous chapter we discussed energy, and
... be more fundamental than Newton's third law of motion, and furthermore Newton's third law of motion can be derived from the principle of conservation of momentum. Furthermore, the principle of conservation of momentum is more general than Newton's third law of motion because the former applies to li ...
... be more fundamental than Newton's third law of motion, and furthermore Newton's third law of motion can be derived from the principle of conservation of momentum. Furthermore, the principle of conservation of momentum is more general than Newton's third law of motion because the former applies to li ...
Open-System Quantum Simulation with Atoms and Ions
... dissipation is well-known from optical pumping of internal electronic states of atoms and laser cooling of motional states. Dissipative preparation of N-particle states by engineering Kraus operators, or quantum jump operators is best illustrated by the conceptually simplest example of “Bell state c ...
... dissipation is well-known from optical pumping of internal electronic states of atoms and laser cooling of motional states. Dissipative preparation of N-particle states by engineering Kraus operators, or quantum jump operators is best illustrated by the conceptually simplest example of “Bell state c ...
Nonlinear electron acceleration by oblique whistler waves - HAL-Insu
... Nonlinear interaction of oblique high-amplitude whistler waves with relativistic electrons involves the Landau resonance besides the fundamental cyclotron one (see review Ref. 6). Even for waves with h < hr , it has been shown that the effect of the parallel electric field can become dominant in the ...
... Nonlinear interaction of oblique high-amplitude whistler waves with relativistic electrons involves the Landau resonance besides the fundamental cyclotron one (see review Ref. 6). Even for waves with h < hr , it has been shown that the effect of the parallel electric field can become dominant in the ...
4.1_simple_harmonic_motion_
... (a) when the springs are connected as in figure (a) calculate the period of oscillations when it is displaced from its equilibrium position and then released. (b) When the springs are connected instead as in figure (b) would the period change. 24. The graph in the figure shows the variation with tim ...
... (a) when the springs are connected as in figure (a) calculate the period of oscillations when it is displaced from its equilibrium position and then released. (b) When the springs are connected instead as in figure (b) would the period change. 24. The graph in the figure shows the variation with tim ...
(DOC, Unknown)
... solution of even a non-relativistic quantum mechanical description, given that “potential energies existing in nature” are considered, leads to solutions, in perfect harmony with the STR. This is to say that, regarding the “internal dynamics” of a quantum mechanical object, “mass” (the mass, in fact ...
... solution of even a non-relativistic quantum mechanical description, given that “potential energies existing in nature” are considered, leads to solutions, in perfect harmony with the STR. This is to say that, regarding the “internal dynamics” of a quantum mechanical object, “mass” (the mass, in fact ...
Field Formulation of Many-Body Quantum Physics {ffmbqp
... As a first step towards developing this powerful theory we shall start from the well-founded Schrödinger theory of nonrelativistic spinless particles. We show that there exists a completely equivalent formulation of this theory in terms of quantum fields. This formulation will serve as a basis for ...
... As a first step towards developing this powerful theory we shall start from the well-founded Schrödinger theory of nonrelativistic spinless particles. We show that there exists a completely equivalent formulation of this theory in terms of quantum fields. This formulation will serve as a basis for ...
Three-dimensional Child–Langmuir law for uniform hot electron
... been a subject of much interest in recent years.8–13 For uniform cold electron emission, a 2D planar classical CL law has been derived analytically,8 which agrees well with the simulation results.9 Enhanced emission near to the beam edges has also been reported in various 2D nonuniform emission mode ...
... been a subject of much interest in recent years.8–13 For uniform cold electron emission, a 2D planar classical CL law has been derived analytically,8 which agrees well with the simulation results.9 Enhanced emission near to the beam edges has also been reported in various 2D nonuniform emission mode ...
F.Y. B.Sc. - Chemistry
... Rutherford model, Electromagnetic spectrum, Bohr’s theory, its limitations and atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s uncertainty Principle and its significance, Schrödinger’s wave equation(derivation not required), significance of ψ and ψ2, quantum numbe ...
... Rutherford model, Electromagnetic spectrum, Bohr’s theory, its limitations and atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s uncertainty Principle and its significance, Schrödinger’s wave equation(derivation not required), significance of ψ and ψ2, quantum numbe ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.